Ninhydrin

| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
2,2-Dihydroxy-1H-indene-1,3(2H)-dione | |
| Other names
2,2-Dihydroxyindane-1,3-dione
1,2,3-Indantrione hydrate | |
| Identifiers | |
3D model (
JSmol ) |
|
| ChEMBL | |
| ChemSpider | |
ECHA InfoCard
|
100.006.926 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C9H6O4 | |
| Molar mass | 178.143 g·mol−1 |
| Appearance | White solid |
| Density | 0.862 g/cm3 |
| Melting point | 250 °C (482 °F; 523 K) (decomposes) |
| 20 g L−1[1] | |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H302, H315, H319, H335 | |
| P261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P312, P321, P330, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
| Safety data sheet (SDS) | External MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Ninhydrin (2,2-dihydroxyindane-1,3-dione) is an organic compound with the formula C6H4(CO)2C(OH)2. It is used to detect ammonia and amines. Upon reaction with these amines, ninhydrin gets converted into deep blue or purple derivatives, which are called Ruhemann's purple. Ninhydrin is most commonly used to detect fingerprints in forensic cases, as the terminal amines of lysine residues in peptides and proteins sloughed off in fingerprints react with ninhydrin.[2][3]
Ninhydrin is a white solid that is soluble in ethanol and acetone.[1] Ninhydrin can be considered as the hydrate of indane-1,2,3-trione.
History
Ninhydrin was discovered in 1910 by the German-English chemist Siegfried Ruhemann (1859–1943).[4][5] In the same year, Ruhemann observed ninhydrin's reaction with amino acids.[6] In 1954, Swedish investigators Oden and von Hofsten proposed that ninhydrin could be used to develop latent fingerprints.[7][8]
Uses
Ninhydrin can be used in Kaiser test to monitor
Ninhydrin is also used in qualitative analysis of proteins. Most of the amino acids, except proline, are hydrolyzed and react with ninhydrin. Also, certain amino acid chains are degraded. Therefore, separate analysis is required for identifying such amino acids that either react differently or do not react with ninhydrin at all. The rest of the amino acids are then quantified colorimetrically after separation by chromatography.
A solution suspected of containing the
Upon reaction with ninhydrin, amino acids undergo

Forensics
A ninhydrin solution is commonly used by
To further enhance the ability of ninhydrin, a solution of
Reactivity
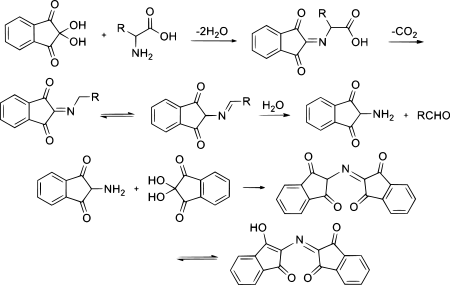
Ninhydrin exists in equilibrium with the triketone indane-1,2,3-trione, which reacts readily with nucleophiles (including water). Whereas for most carbonyl compounds, a carbonyl form is more stable than a product of water addition (hydrate), ninhydrin forms a stable hydrate of the central carbon because of the destabilizing effect of the adjacent carbonyl groups.
To generate the ninhydrin chromophore [2-(1,3-dioxoindan-2-yl)iminoindane-1,3-dione], the amine must condense to give a Schiff base. The reaction of ninhydrin with secondary amines gives an iminium salt, which is also coloured, generally being yellow–orange.
Effects on health
Ninhydrin may cause allergic, IgE-mediated rhinitis and asthma.[17] A case has been described in which a 41 year old forensic laboratory worker working with Ninhydrin developed rhinitis and respiratory difficulty. Her specific IgE levels were found almost doubled.[17]
See also
References
- ^ a b Chemicals and reagents, 2008–2010, Merck
- ^ "Fingerprinting Analysis". Bergen County Technical Schools. June 2003. Archived from the original on 13 June 2007.
- ISBN 9780471238966.
- .
- .
- .
- S2CID 4187222.
- ^ Oden, Svante. "Process of Developing Fingerprints". U.S. Patent no. 2,715,571 (filed: 27 September 1954; issued: 16 August 1955).
- PMID 5443684.
- .
- S2CID 23508811.
- PMID 14979690.)
{{cite journal}}: CS1 maint: multiple names: authors list (link - S2CID 6245255.)
{{cite journal}}: CS1 maint: multiple names: authors list (link - ISBN 0862522307
- S2CID 214732288.
- PMID 26385717.
- ^ PMID 9272939.