Photoperiodism
Photoperiodism is the physiological reaction of organisms to the length of light or a dark period. It occurs in plants and animals. Plant photoperiodism can also be defined as the developmental responses of plants to the relative lengths of light and dark periods. They are classified under three groups according to the photoperiods: short-day plants, long-day plants, and day-neutral plants.
In animals photoperiodism (sometimes called seasonality) is the suite of physiological changes that occur in response to changes in day length. This allows animals to respond to a temporally changing environment associated with changing seasons as the earth orbits the sun.
Plants
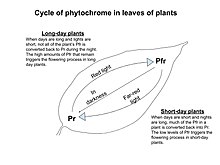
Many flowering plants (angiosperms) use a circadian rhythm together with photoreceptor protein, such as phytochrome or cryptochrome,[1] to sense seasonal changes in night length, or photoperiod, which they take as signals to flower. In a further subdivision, obligate photoperiodic plants absolutely require a long or short enough night before flowering, whereas facultative photoperiodic plants are more likely to flower under one condition.
Cryptochromes are another type of photoreceptor that is important in photoperiodism. Cryptochromes absorb blue light and UV-A. Cryptochromes entrain the circadian clock to light.[6] It has been found that both cryptochrome and phytochrome abundance relies on light and the amount of cryptochrome can change depending on day-length. This shows how important both of the photoreceptors are in regards to determining day-length.[7]
In 1920, W. W. Garner and H. A. Allard published their discoveries on photoperiodism and felt it was the length of daylight that was critical,[1][8] but it was later discovered that the length of the night was the controlling factor.[9][10] Photoperiodic flowering plants are classified as long-day plants or short-day plants even though night is the critical factor because of the initial misunderstanding about daylight being the controlling factor. Along with long-day plants and short-day plants, there are plants that fall into a "dual-day length category". These plants are either long-short-day plants (LSDP) or short-long-day plants (SLDP). LSDPs flower after a series of long days followed by short days whereas SLDPs flower after a series of short days followed by long days.[11] Each plant has a different length critical photoperiod, or critical night length.[1]
Modern biologists believe[12] that it is the coincidence of the active forms of phytochrome or cryptochrome, created by light during the daytime, with the rhythms of the circadian clock that allows plants to measure the length of the night. Other than flowering, photoperiodism in plants includes the growth of stems or roots during certain seasons and the loss of leaves. Artificial lighting can be used to induce extra-long days.[1]
Long-day plants

Long-day plants flower when the night length falls below their critical photoperiod.[13] These plants typically flower during late spring or early summer as days are getting longer. In the northern hemisphere, the longest day of the year (summer solstice) is on or about 21 June.[14] After that date, days grow shorter (i.e. nights grow longer) until 21 December (the winter solstice). This situation is reversed in the southern hemisphere (i.e., longest day is 21 December and shortest day is 21 June).[1][8]
Some long-day obligate plants are:
Some long-day facultative plants are:
Short-day plants

Short-day (also called long-night) plants flower when the night lengths exceed their critical photoperiod.[15] They cannot flower under short nights or if a pulse of artificial light is shone on the plant for several minutes during the night; they require a continuous period of darkness before floral development can begin. Natural nighttime light, such as moonlight or lightning, is not of sufficient brightness or duration to interrupt flowering.[1][8]
Short-day plants flower as days grow shorter (and nights grow longer) after 21 June in the northern hemisphere, which is during summer or fall. The length of the dark period required to induce flowering differs among species and varieties of a species.
Photoperiodism affects flowering by inducing the shoot to produce floral buds instead of leaves and lateral buds.
Some short-day facultative plants are:[16]
- Kenaf ( Hibiscus cannabinus)
- Marijuana(Cannabis)
- Cotton (Gossypium)
- Rice (Oryza)
- Sorghum (Sorghum bicolor)
- Green gram(Mung bean, Vigna radiata)
- Soybeans[17] (Glycine max)
Day-neutral plants
Day-neutral plants, such as
Animals
Daylength, and thus knowledge of the season of the year, is vital to many animals. A number of biological and behavioural changes are dependent on this knowledge. Together with temperature changes, photoperiod provokes changes in the color of fur and feathers,
In insects, sensitivity to photoperiod has been proven to be initiated by photoreceptors located in the brain.[19][20] Photoperiod can affect insects at different life stages, serving as an environmental cue for physiological processes such as diapause induction and termination, and seasonal morphs.[21] In the water strider Aquarius paludum, for instance, photoperiod conditions during nymphal development have been shown to trigger seasonal changes in wing frequency and also induce diapause, although the threshold critical day lengths for the determination of both traits diverged by about an hour.[22] In Gerris buenoi, another water strider species, photoperiod has also been shown to be the cause of wing polyphenism,[23] although the specific daylengths changed between species, suggesting that phenotypic plasticity in response to photoperiod has evolved even between relatively closely related species.
The singing frequency of birds such as the
In mammals, daylength is registered in the
Some mammals are highly seasonal. The view has been expressed that humans' seasonality is largely believed to be evolutionary baggage.[25][relevant?]. Human birth rate varies throughout the year, and the peak month of births appears to vary by latitude.[26] Seasonality in human birth rate appears to have largely decreased since the industrial revolution.[26][27]
Other organisms
Photoperiodism has also been demonstrated in other organisms besides plants and animals. The fungus Neurospora crassa as well as the dinoflagellate Lingulodinium polyedra and the unicellular green alga Chlamydomonas reinhardtii have been shown to display photoperiodic responses.[28][29][30]
See also
References
- ^ ISBN 978-0-7637-2134-3.
- PMID 11279228.
- PMID 24220656.
- PMID 10806223.
- ISBN 978-0-374-28873-0.
- PMID 15892880.
- PMID 12578985.
- ^ ISBN 978-0-88192-655-2.
- S2CID 84084837.
- S2CID 83682483.
- ISBN 978-1-60535-353-1.
- PMID 19675157.
- ISBN 978-1-111-58068-1.
- ISBN 978-0-7535-2311-7.
- ISBN 978-0-7872-9008-5.
- ISBN 978-0-521-42524-7.
- ^ Purcell LC, Salmeron M, Ashlock L (2014). "Chapter 2" (PDF). Arkansas Soybean Production Handbook - MP197. Little Rock, Arkansas: University of Arkansas Cooperative Extension Service. pp. 5–7. Retrieved 21 February 2016.
- ISBN 978-0-19-968126-6.
- ^ Claret J (1966). "Recherche du centre photorecepteur lors de l'induction de la diapause chez Pieris brassicae L.". Comptes Rendus de l'Académie des Sciences. 262: 553–556.
- PMID 6592591.
- S2CID 85249708.
- S2CID 39616943.
- PMID 35473377.
- ^ Nelson RJ (2005). An Introduction to Behavioral Endocrinology. Sunderland, MA: Sinauer Associates. p. 189.
- ^ Foster R, Williams R (5 December 2009). "Extra-retinal photo receptors" (Interview). Science Show. ABC Radio National. Retrieved 2010-05-28.
...we have the evolutionary baggage of showing seasonality but we're not entirely sure what the mechanism is.
- ^ PMID 24695423.
- S2CID 25886221.
- ^ Tan Y, Merrow M, Roenneberg T. Photoperiodism in Neurospora crassa. J Biol Rhythms. 2004 Apr;19(2):135-43. https://doi.org/10.1177/0748730404263015. PMID 15038853.
- ^ Suzuki, L., Johnson, C. Photoperiodic control of germination in the unicell Chlamydomonas. Naturwissenschaften 89, 214–220 (2002). https://doi.org/10.1007/s00114-002-0302-6
- ^ Ivonne Balzer, Rüdiger Hardeland ,Photoperiodism and Effects of Indoleamines in a Unicellular Alga, Gonyaulax polyedra.Science253,795-797(1991).https://doi.org/10.1126/science.1876838
Further reading
- Fosket DE (1994). Plant Growth & Development, A Molecular Approach. San Diego: Academic Press. p. 495.
- Thomas B, Vince-Prue D (1997). Photoperiodism in plants (2nd ed.). Academic Press.

