Polyfluorene
| |
| Identifiers | |
|---|---|
| ChemSpider |
|
| Properties | |
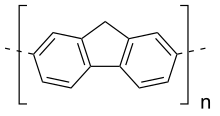
| (C13H8)n | |
| Molar mass | Variable |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Polyfluorene is a polymer with formula (C13H8)n, consisting of fluorene units linked in a linear chain — specifically, at carbon atoms 2 and 7 in the standard fluorene numbering. It can also be described as a chain of benzene rings linked in para positions (a polyparaphenylene) with an extra methylene bridge connecting every pair of rings.
The two benzene rings in each unit make polyfluorene an
When spoken about as a class, polyfluorenes are derivatives of this polymer, obtained by replacing some of the hydrogen atoms by other
History
Fluorene, the repeat unit in polyfluorene derivatives, was isolated from coal tar and discovered by Marcellin Berthelot prior to 1883.[1][2][3] Its name originates from its interesting fluorescence (and not to fluorine, which is not one of its elements).
Fluorene became the subject of chemical-structure related
The physical properties of the fluorene molecule were recognizably desirable for polymers; as early as the 1970s researchers began incorporating this moiety into polymers. For instance, because of fluorene’s rigid, planar shape a polymer containing fluorene was shown to exhibit enhanced thermo-mechanical stability.[6] However, more promising was integrating the optoelectronic properties of fluorene into a polymer. Reports of the
As interest in conducting plastics grew, fluorene again found application. The
Properties



Polyfluorenes are an important class of polymers which have the potential to act as both electroactive and
Interest in polyfluorene derivatives has increased because of their high photoluminescence quantum efficiency, high thermal stability, and their facile color tunability, obtained by introducing low-band-gap co-monomers. Research in this field has increased significantly due to its potential application in tuning
Another important quality of polyfluorenes is their
Challenges associated with polyfluorenes
Polyfluorenes often show both
Polyfluorenes can also undergo decomposition. There are two known ways in which decomposition can occur. The first involves the oxidation of the polymer that leads to the formation of an aromatic ketone, quenching the fluorescence. The second decomposition process results in aggregation leading to a red-shifted fluorescence, reduced intensity, exciton migration and relaxation through excimers.[23]
Researchers have attempted to eliminate excimer formation and enhance the efficiency of polyfluorenes by copolymerizing polyfluorene with
Aggregation has also been combated by varying the chemical structure. For example, when conjugated polymers aggregate, which is natural in the solid state, their emission can be self-quenched, reducing luminescent quantum yields and reducing luminescent device performance. In opposition to this tendency, researchers have used tri-functional monomers to create highly branched polyfluorenes which do not aggregate due to the bulkiness of the substituents. This design strategy has achieved luminescent quantum yields of 42% in the solid state.[25] This solution reduces the ease of processability of the material because branched polymers have increased chain entanglement and poor solubility.
Another problem commonly encountered by polyfluorenes is an observed broad green, parasitic emission which detracts from the color purity and efficiency needed for an OLED.[18][19][26] Initially attributed to excimer emission, this green emission has been shown to be due to the formation of ketone defects along the fluorene polymer backbone (oxidation of the nine position on the monomer) when there are incomplete substitution at the nine positions of the fluorene monomer.[18] Routes to combat this involve ensuring full substitution of the monomer’s active site, or including aromatic substituents.[18] These solutions may present structures that lack optimal bulkiness or may be synthetically difficult.
Synthesis and design
Conjugated polymers, such as polyfluorene, can be designed and synthesized with different properties for a wide variety of applications.[19] The color of the molecules can be designed through synthetic control over the electron donating or withdrawing character of the substituents on fluorene or the comonomers in polyfluorene.[20][27][28]

Solubility of the polymers are important because solution state processing is very common. Since conjugated polymers, with their planar structure, tend to aggregate, bulky side chains are added (to the 9 position of fluorene) to increase the solubility of the polymer.
Oxidative polymerization
The earliest polymerizations of fluorene were
Cross coupling polymerizations
The design of polymeric properties requires great control over the structure of the polymer. For instance, low band gap polymers require regularly alternating electron donating and electron accepting monomers.[11][18] More recently, many popular


Design
Modern coupling chemistries allow other properties of polyfluorenes to be controlled through implementation of complex molecular designs.

The above polymer structure pictured has excellent photoluminescent quantum yields (partly due to its fluorene monomer) excellent stability (due to its oxadiazole comonomer) good solubility (due to its many and branched alkyl side chains) and has an amine functionalized side chain for ease of tethering to other molecules or to a substrate.[13] The luminescent color of polyfluorenes can be changed, for example, (from blue to green-yellow) by adding functional groups which participate in excited state intramolecular proton transfer. Exchanging the alkoxy side chains for alcohol side groups allows for energy dissipation (and a red-shift in emission) through reversible transfer of a proton from the alcohol to the nitrogen (on the oxadiazole). These complicated molecular structures were engineered to have these properties and were only able to be realized through careful control of their ordering and side group functionality.
Applications
Organic light-emitting diodes (OLEDs)
In recent years many industrial efforts have focused on tuning the color of lights using polyfluorenes. It was found that by doping green or red emitting materials into polyfluorenes one could tune the color emitted by the polymers. Since polyfluorene homopolymers emit higher energy blue light, they can transfer energy via Förster resonance energy transfer (FRET) to lower energy emitters. In addition to doping, color of polyfluorenes can be tuned by copolymerizing the fluorene monomers with other low band gap monomers. Researchers at the Dow Chemical Company synthesized several fluorene-based copolymers by alternating copolymerization using 5,5-dibromo-2,2-bithiophene which showed yellow emission and 4,7-dibromo-2,1,3-benzothiadiazole, which showed green emission. Other copolymerizations are also suitable; researchers at IBM performed random copolymerization of fluorene with 3,9(10)-dibromoperylene,4,4-dibromo-R-cyanostilbene, and 1,4-bis(2-(4-bromophenyl)-1-cyanovinyl)-2-(2-ethylhexyl)-5-methoxybenzene. Only a small amount of the co-monomer, approximately 5%, was needed to tune the emission of the polyfluorene from blue to yellow. This example further illustrates that by introducing monomers that have a lower band gap than the fluorene monomer, one can tune the color that is emitted by the polymer.[20]
Substitution at the nine position with various moieties has also been examined as a means to control the color emitted by polyfluorene. In the past researchers have tried putting
Polymer solar cells
Polyfluorenes are also used in
The voltage of polymer solar cells has also been increased through the design of polyfluorenes. These devices are typically produced by blending electron accepting and electron donating molecules which help separate charge to produce power. In polymer blend solar cells, the voltage produced by the device is determined by the difference between the electron donating polymer’s
Typical polymer solar cells utilize fullerene molecules as electron acceptors because of their low LUMO energy level (high electron affinity). However the tunability of polyfluorenes allows their LUMO to be lowered to a level appropriate for use as an electron acceptor. Thus, polyfluorene copolymers have also been used in polymer:polymer blend solar cells, where their electron accepting, electron conducting and light absorbing properties permit device performance.[39][40]
References
- ^ .
- ^ "Fluorene". Ann. Chim. Phys. 12 (4): 222. 1867.
- .
- .
- .
- .
- ^ .
- ^ .
- ^ .
- ^ .
- ^ .
- doi:10.1063/1.126991.
- ^ .
- ^ Dillow, Clay (2010-06-07). "Laser Sensor Can See Explosives' Vapor Trails Even at Extremely Low Concentrations". Popular Science. Bonnier Corporation. Retrieved 2011-03-28.
- .
- S2CID 4184730.
- ISBN 9780471660064.
- ^ ISBN 978-3-540-68733-7.
- ^ .
- ^ .
- ^ .
- .
- .
- ISSN 0935-9648.
- ^ .
- .
- ^ .
- ^ .
- .
- S2CID 120685112.
- .
- .
- .
- .
- .
- ^ .
- ^ PMID 19788319.
- S2CID 13848611.
- S2CID 5272193.
- doi:10.1002/pip.796.
Further reading
- Barford, William (2005). Electronic and Optical Properties of Conjugated Polymers. Oxford University Press. ISBN 0-19-852680-6.
- Hamilton, Michael (2005). Polyfluorene-based organic field-effect transistors (Thesis dissertation). ISBN 0-542-36494-8.
- Hong, Meng; Li, Zhigang (2007). Organic Light-emitting Materials and Devices. CRC Press. ISBN 978-1-57444-574-9.
- Lee, Shu-Jen (2004). Organic Polymer Light-emitting Devices: Optical Modeling, Engineering and Evaluation of Opto-electronic Properties (Thesis dissertation). ISBN 978-0-496-09054-9.
