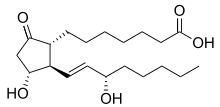
Prostaglandin E1
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Caverject, Muse, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a695022 |
| License data |
|
Intravenous | |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
JSmol) | |
| |
| |
| | |
Prostaglandin E1 (PGE1) is a naturally occurring prostaglandin and is also used as a medication (alprostadil).[2]
In infants with
Common side effects when given to babies include decreased breathing,
Prostaglandin E1 was isolated in 1957 and approved for medical use in the United States in 1981.[2][5] It is on the World Health Organization's List of Essential Medicines.[6]
Biosynthesis
Prostaglandin E1 is biosynthesized on an as-needed basis from dihomo-γ-linolenic acid (an omega-6 fatty acid) in healthy humans without coronary artery disease[7] and/or a genetic disorder.
Medical uses
Patent ductus arteriosus
Alprostadil is also used in maintaining a
Sexual dysfunction
Alprostadil is sold in the United States as
Alprostadil is also available as a generic. It must be mixed by a
This section contains content that is written like an advertisement. (May 2021) ) |
The compound has been made into an applicable topical cream form known as Vitaros,[11] made by Takeda UK Ltd., it contains either 200 or 300 μg of alprostadil in 100mg of cream. The tip of the device is placed in the urethral meatus, and the cream is delivered into the urethra.
Off-brand Uses, Interactions
Clinical trials for the treatment showed positive results in around 3,000 men that it was tested on; it is said to be usable by men with diabetes or heart problems and those who have undergone a prostatectomy.[12] It has no known interactions with food, alcohol or other medications.
Misoprostol is another synthetic prostaglandin E1 analog used to prevent gastric ulcers when taken on a continuous basis,[13] to treat missed miscarriage,[14] to induce labor,[15] and to induce abortion.[16]
Critical limb ischemia
Prostanoids, including alprostadil, do not reduce the risk of limb amputation but may offer a slight improvement in rest-pain and leg ulcer healing in persons with critical limb ischemia.[17]
Contrast-induced nephropathy
Preventative administration of alprostadil may reduce the risk of kidney injury (specifically contrast-induced nephropathy) in persons having cardiac angiography or percutaneous coronary intervention.[18][19]
Adverse effects
- Accidental injury (Muse only)
- Apnea
- Bleeding:
- Cerebral
- Urethral
- Bradycardia
- Cardiac arrest
- Congestive heart failure
- Cortical proliferation of long bones
- Diarrhea
- Disseminated intravascular coagulation
- Edema
- Fever
- Flushing
- Hyperemia
- Hypotension
- Injection-site haematoma
- Injection-site ecchymosis (Caverject only)
- Pain:
- Back
- Pelvic
- Penile
- Testicular (Muse only)
- Urethral
- Prolonged erection
- Penile fibrosis
- Second-degree heart block
- Seizures
- Sepsis
- Shock
- Spasm of right ventricle infundibulum
- Supraventricular tachycardia
- Tachycardia
- Ventricular fibrillation
- Urethral burning
- Uterine rupture
References
- FDA. Retrieved 22 October 2023.
- ^ a b c d e f "Alprostadil". The American Society of Health-System Pharmacists. Archived from the original on 16 January 2017. Retrieved 8 January 2017.
- ISBN 9780470750353. Archivedfrom the original on 13 January 2017.
- ISBN 9780857111562.
- ISBN 9780470015520. Archivedfrom the original on 13 January 2017.
- hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- PMID 23735361.)
{{cite journal}}: CS1 maint: multiple names: authors list (link - ^ "Muse Suppository - Facts and Comparisons". Drugs.com. Archived from the original on 19 January 2013. Retrieved 4 January 2013.
- ^ "Edex - Facts and Comparisons". Drugs.com. Archived from the original on 26 October 2012.
- ^ "Caverject - Facts and Comparisons". Drugs.com. Archived from the original on 26 October 2012.
- ^ "Vitaros 3 mg/g cream - Summary of Product Characteristics". Medicines.org.uk. Archived from the original on 11 February 2015.
- ^ "Vitaros- New Erectile Dysfunction Topical Treatment". Meds4All.co.uk. Archived from the original on 11 February 2015.
- PMID 1435885.
- PMID 28490770.
- PMID 30907996.
- ^ "Medical abortion". Mayo Clinic. Retrieved 28 April 2022.
- PMID 29318581.
- PMID 27861357.
- S2CID 58950588.
