Rydberg matter
This article has multiple issues. Please help improve it or discuss these issues on the talk page. (Learn how and when to remove these messages)
|
Rydberg matter
Physical
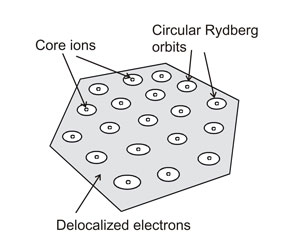

Rydberg matter consists of usually
Lifetime

Due to reasons still debated by the physics community because of the lack of methods to observe clusters,[27] Rydberg matter is highly stable against disintegration by emission of radiation; the characteristic lifetime of a cluster at n = 12 is 25 seconds.[26][28] Reasons given include the lack of overlap between excited and ground states, the forbidding of transitions between them and exchange-correlation effects hindering emission through necessitating tunnelling[23] that causes a long delay in excitation decay.[25] Excitation plays a role in determining lifetimes, with a higher excitation giving a longer lifetime;[26] n = 80 gives a lifetime comparable to the age of the Universe.[29]
Excitations
| n | d (nm) | D (cm−3) |
|---|---|---|
| 1 | 0.153 | 2.8×1023 |
| 4 | 2.45 | |
| 5 | 3.84 | |
| 6 | 5.52 | |
| 10 | 15.3 | 2.8×1017 |
| 40 | 245 | |
| 80 | 983 | |
| 100 | 1534 | 2.8×1011 |
In ordinary metals, interatomic distances are nearly constant through a wide range of temperatures and pressures; this is not the case with Rydberg matter, whose distances and thus properties vary greatly with excitations. A key variable in determining these properties is the principal quantum number n that can be any integer greater than 1; the highest values reported for it are around 100.[29][30] Bond distance d in Rydberg matter is given by
where a0 is the Bohr radius. The approximate factor 2.9 was first experimentally determined, then measured with rotational spectroscopy in different clusters.[16] Examples of d calculated this way, along with selected values of the density D, are given in the adjacent table.
Condensation
Like bosons that can be condensed to form Bose–Einstein condensates, Rydberg matter can be condensed, but not in the same way as bosons. The reason for this is that Rydberg matter behaves similarly to a gas, meaning that it cannot be condensed without removing the condensation energy; ionisation occurs if this is not done. All solutions to this problem so far involve using an adjacent surface in some way, the best being evaporating the atoms of which the Rydberg matter is to be formed from and leaving the condensation energy on the surface.[31] Using caesium atoms, graphite-covered surfaces and thermionic converters as containment, the work function of the surface has been measured to be 0.5 eV,[32] indicating that the cluster is between the ninth and fourteenth excitation levels.[25]
See also
The overview[33] provides information on Rydberg matter and possible applications in developing clean energy, catalysts, researching space phenomena, and usage in sensors.
Disputed
The research claiming to create ultradense hydrogen Rydberg matter (with interatomic spacing of ~2.3 pm: many orders of magnitude less than in most solid matter) is disputed:[34]
″The paper of Holmlid and Zeiner-Gundersen makes claims that would be truly revolutionary if they were true. We have shown that they violate some fundamental and very well established laws in a rather direct manner. We believe we share this scepticism with most of the scientific community. The response to the theories of Holmlid is perhaps most clearly reflected in the reference list of their article. Out of 114 references, 36 are not coauthored by Holmlid. And of these 36, none address the claims made by him and his co-authors. This is so much more remarkable because the claims, if correct, would revolutionize quantum science, add at least two new forms of hydrogen, of which one is supposedly the ground state of the element, discover an extremely dense form of matter, discover processes that violate baryon number conservation, in addition to solving humanity’s need for energy practically in perpetuity.″
References
- .
- ^ É.A. Manykin; M.I. Ozhovan; P.P. Poluéktov (1980). "Transition of an excited gas to a metallic state". Sov. Phys. Tech. Phys. Lett. 6: 95.
- ^ Bibcode:1981SPhD...26..974M.)
{{cite journal}}: CS1 maint: multiple names: authors list (link - S2CID 122574536.
- .
- ^
S. Badiei & L. Holmlid (2006). "Experimental studies of fast fragments of H Rydberg matter". S2CID 120897660.
- ^ .
- .
- S2CID 120594462.
- ^ .
- PMID 9897204.
- ISBN 978-0-7923-0804-1.
- S2CID 95032480.
- S2CID 120239230.
- .
- ^ ISSN 0022-2860.
- ISSN 0039-6028.
- S2CID 93258712.
- ^ ISSN 0301-0104.
- ISSN 0301-0104.
- S2CID 123663484.
- ISSN 0032-0633.
- ^ Bibcode:1983JETP...57..256M.
- ISSN 0377-0486.
- ^ Bibcode:1992JETP...75..602M.
- ^ Bibcode:1994JETP...78...27M.
- S2CID 250782651.
- ISSN 0370-1573.
- ^ a b L. Holmlid, "Redshifts in space caused by stimulated Raman scattering in cold intergalactic Rydberg Matter with experimental verification". J. Exp. Theor. Phys. JETP 100 (2005) 637–644.
- .
- ISSN 0021-9606.
- ISSN 0039-6028.
- ^ Aasen, T.H., Zeiner-Gundersen, D.H., Zeiner-Gundersen, S. et al. A Condensed Excited (Rydberg) Matter: Perspective and Applications. J. Clust. Sci. (2021), https://doi.org/10.1007/s10876-021-02031-6, 14 p.
- arXiv:2207.08133.