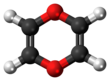
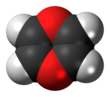
1,4-Dioxin
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1,4-Dioxine[1] | |||
| Systematic IUPAC name
1,4-Dioxacyclohexa-2,5-diene | |||
| Other names
1,4-Dioxin
Dioxin p-Dioxin 1,4-Dioxa[6]annulene | |||
| Identifiers | |||
3D model (
JSmol ) |
|||
| ChEBI | |||
| ChemSpider | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C4H4O2 | |||
| Molar mass | 84.07 g/mol | ||
| Appearance | Colorless liquid | ||
| Boiling point | 75 °C (167 °F; 348 K) | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
highly flammable | ||
| Related compounds | |||
Related compounds
|
dibenzodioxin | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
1,4-Dioxin (also referred as dioxin or p-dioxin) is a heterocyclic, organic, non-aromatic[2] compound with the chemical formula C4H4O2. There is an isomeric form of 1,4-dioxin, 1,2-dioxin (or o-dioxin). 1,2-Dioxin is very unstable due to its peroxide-like characteristics.
The term "dioxin" is most commonly used for a family of derivatives of dioxin, known as polychlorinated dibenzodioxins (PCDDs).
Preparation
1,4-Dioxin can be prepared by cycloaddition, namely by the Diels–Alder reaction of furan and maleic anhydride. The adduct formed has a carbon-carbon double bond, which is converted to an epoxide. The epoxide then undergoes a retro-Diels–Alder reaction, forming 1,4-dioxin and regenerating maleic anhydride.[3]
Derivatives
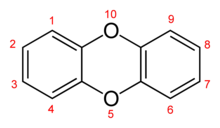
The word "dioxin" can refer in a general way to compounds which have a dioxin core skeletal structure with substituent molecular groups attached to it. For example, dibenzo-1,4-dioxin is a compound whose structure consists of two benzo- groups fused onto a 1,4-dioxin ring.
Polychlorinated dibenzodioxins
Because of their extreme importance as environmental pollutants, current scientific literature uses the name dioxins commonly for simplification to denote the chlorinated derivatives of dibenzo-1,4-dioxin, more precisely the
PCDDs are formed through combustion, chlorine bleaching and manufacturing processes.[4] The combination of heat and chlorine creates dioxin.[4] Since chlorine is often a part of the Earth's environment, natural ecological activity such as volcanic activity and forest fires can lead to the formation of PCDDs.[4] Nevertheless, PCDDs are mostly produced by human activity.[4]
Famous PCDD exposure cases include
Polychlorinated dibenzofurans are a related class compounds to PCDDs which are often included within the general term "dioxins".
References
- ISBN 978-0-85404-182-4.
- ^ Science of Synthesis: Houben-Weyl Methods of Molecular Transformations Vol. 16: Six-Membered Hetarenes with Two Identical Heteroatoms
- .
- ^ a b c d "Dioxin Information". Department of Environmental Protection, State of Maine. 2005. Archived from the original on 2009-06-15. Retrieved 2008-08-10.