Diels–Alder reaction
| Diels–Alder reaction | |
|---|---|
| Reaction type | Cycloaddition |
| Identifiers | |
| Organic Chemistry Portal | diels-alder-reaction |
| RSC ontology ID | RXNO:0000006 |
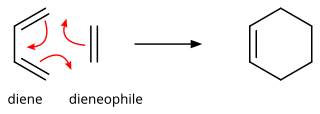
In organic chemistry, the Diels–Alder reaction is a chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene derivative. It is the prototypical example of a pericyclic reaction with a concerted mechanism. More specifically, it is classified as a thermally-allowed [4+2] cycloaddition with Woodward–Hoffmann symbol [π4s + π2s]. It was first described by Otto Diels and Kurt Alder in 1928. For the discovery of this reaction, they were awarded the Nobel Prize in Chemistry in 1950. Through the simultaneous construction of two new carbon–carbon bonds, the Diels–Alder reaction provides a reliable way to form six-membered rings with good control over the regio- and stereochemical outcomes.[1][2] Consequently, it has served as a powerful and widely applied tool for the introduction of chemical complexity in the synthesis of natural products and new materials.[3][4] The underlying concept has also been applied to π-systems involving heteroatoms, such as carbonyls and imines, which furnish the corresponding heterocycles; this variant is known as the hetero-Diels–Alder reaction. The reaction has also been generalized to other ring sizes, although none of these generalizations have matched the formation of six-membered rings in terms of scope or versatility. Because of the negative values of ΔH° and ΔS° for a typical Diels–Alder reaction, the microscopic reverse of a Diels–Alder reaction becomes favorable at high temperatures, although this is of synthetic importance for only a limited range of Diels-Alder adducts, generally with some special structural features; this reverse reaction is known as the retro-Diels–Alder reaction.[5]
Mechanism
The reaction is an example of a concerted pericyclic reaction.
A consideration of the reactants' frontier molecular orbitals (FMO) makes plain why this is so. (The same conclusion can be drawn from an orbital correlation diagram or a Dewar-Zimmerman analysis.) For the more common "normal" electron demand Diels–Alder reaction, the more important of the two HOMO/LUMO interactions is that between the electron-rich diene's ψ2 as the highest occupied molecular orbital (HOMO) with the electron-deficient dienophile's π* as the lowest unoccupied molecular orbital (LUMO). However, the HOMO–LUMO energy gap is close enough that the roles can be reversed by switching electronic effects of the substituents on the two components. In an inverse (reverse) electron-demand Diels–Alder reaction, electron-withdrawing substituents on the diene lower the energy of its empty ψ3 orbital and electron-donating substituents on the dienophile raise the energy of its filled π orbital sufficiently that the interaction between these two orbitals becomes the most energetically significant stabilizing orbital interaction. Regardless of which situation pertains, the HOMO and LUMO of the components are in phase and a bonding interaction results as can be seen in the diagram below. Since the reactants are in their ground state, the reaction is initiated thermally and does not require activation by light.[8]

The "prevailing opinion"[9][10][11][12] is that most Diels–Alder reactions proceed through a concerted mechanism; the issue, however, has been thoroughly contested. Despite the fact that the vast majority of Diels–Alder reactions exhibit stereospecific, syn addition of the two components, a diradical intermediate has been postulated[7] (and supported with computational evidence) on the grounds that the observed stereospecificity does not rule out a two-step addition involving an intermediate that collapses to product faster than it can rotate to allow for inversion of stereochemistry.
There is a notable rate enhancement when certain Diels–Alder reactions are carried out in polar organic solvents such as
The geometry of the diene and dienophile components each propagate into stereochemical details of the product. For
Regioselectivity
Frontier molecular orbital theory has also been used to explain the regioselectivity patterns observed in Diels–Alder reactions of substituted systems. Calculation of the energy and orbital coefficients of the components' frontier orbitals[17] provides a picture that is in good accord with the more straightforward analysis of the substituents' resonance effects, as illustrated below.
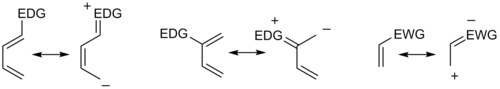
In general, the regioselectivity found for both normal and inverse electron-demand Diels–Alder reaction follows the ortho-para rule, so named, because the cyclohexene product bears substituents in positions that are analogous to the ortho and para positions of disubstituted arenes. For example, in a normal-demand scenario, a diene bearing an electron-donating group (EDG) at C1 has its largest HOMO coefficient at C4, while the dienophile with an electron withdrawing group (EWG) at C1 has the largest LUMO coefficient at C2. Pairing these two coefficients gives the "ortho" product as seen in case 1 in the figure below. A diene substituted at C2 as in case 2 below has the largest HOMO coefficient at C1, giving rise to the "para" product. Similar analyses for the corresponding inverse-demand scenarios gives rise to the analogous products as seen in cases 3 and 4. Examining the canonical mesomeric forms above, it is easy to verify that these results are in accord with expectations based on consideration of electron density and polarization.

In general, with respect to the energetically most well-matched HOMO-LUMO pair, maximizing the interaction energy by forming bonds between centers with the largest frontier orbital coefficients allows the prediction of the main regioisomer that will result from a given diene-dienophile combination.[8] In a more sophisticated treatment, three types of substituents (Z withdrawing: HOMO and LUMO lowering (CF3, NO2, CN, C(O)CH3), X donating: HOMO and LUMO raising (Me, OMe, NMe2), C conjugating: HOMO raising and LUMO lowering (Ph, vinyl)) are considered, resulting in a total of 18 possible combinations. The maximization of orbital interaction correctly predicts the product in all cases for which experimental data is available. For instance, in uncommon combinations involving X groups on both diene and dienophile, a 1,3-substitution pattern may be favored, an outcome not accounted for by a simplistic resonance structure argument.[18] However, cases where the resonance argument and the matching of largest orbital coefficients disagree are rare.
Stereospecificity and stereoselectivity
Diels–Alder reactions, as concerted cycloadditions, are


Diels–Alder reactions in which adjacent stereocenters are generated at the two ends of the newly formed single bonds imply two different possible stereochemical outcomes. This is a
In cases where the dienophile has a single electron-withdrawing / conjugating substituent, or two electron-withdrawing / conjugating substituents cis to each other, the outcome can often be predicted. In these "normal demand" Diels–Alder scenarios, the endo transition state is typically preferred, despite often being more sterically congested. This preference is known as the Alder endo rule. As originally stated by Alder, the transition state that is preferred is the one with a "maximum accumulation of double bonds." Endo selectivity is typically higher for rigid dienophiles such as

Often, as with highly substituted dienes, very bulky dienophiles, or reversible reactions (as in the case of furan as diene), steric effects can override the normal endo selectivity in favor of the exo isomer.
The diene
The diene component of the Diels–Alder reaction can be either open-chain or cyclic, and it can host many different types of substituents.[6] It must, however, be able to exist in the s-cis conformation, since this is the only conformer that can participate in the reaction. Though butadienes are typically more stable in the s-trans conformation, for most cases energy difference is small (~2–5 kcal/mol).[26]
A bulky substituent at the C2 or C3 position can increase reaction rate by destabilizing the s-trans conformation and forcing the diene into the reactive s-cis conformation. 2-tert-butyl-buta-1,3-diene, for example, is 27 times more reactive than simple butadiene.[6][27] Conversely, a diene having bulky substituents at both C2 and C3 is less reactive because the steric interactions between the substituents destabilize the s-cis conformation.[27]
Dienes with bulky terminal substituents (C1 and C4) decrease the rate of reaction, presumably by impeding the approach of the diene and dienophile.[28]
An especially reactive diene is 1-methoxy-3-trimethylsiloxy-buta-1,3-diene, otherwise known as Danishefsky's diene.[29] It has particular synthetic utility as means of furnishing α,β–unsaturated cyclohexenone systems by elimination of the 1-methoxy substituent after deprotection of the enol silyl ether. Other synthetically useful derivatives of Danishefsky's diene include 1,3-alkoxy-1-trimethylsiloxy-1,3-butadienes (Brassard dienes)[30] and 1-dialkylamino-3-trimethylsiloxy-1,3-butadienes (Rawal dienes).[31] The increased reactivity of these and similar dienes is a result of synergistic contributions from donor groups at C1 and C3, raising the HOMO significantly above that of a comparable monosubstituted diene.[3]

Unstable (and thus highly reactive) dienes can be synthetically useful, e.g. o-
The dienophile
In a normal demand Diels–Alder reaction, the dienophile has an electron-withdrawing group in conjugation with the alkene; in an inverse-demand scenario, the dienophile is conjugated with an electron-donating group.
Variants on the classical Diels–Alder reaction
Hetero-Diels–Alder
Diels–Alder reactions involving at least one
Lewis acid activation
Recent studies, however, have shown that this rationale behind Lewis acid-catalyzed Diels–Alder reactions is incorrect.[39][40][41][42] It is found that Lewis acids accelerate the Diels–Alder reaction by reducing the destabilizing steric Pauli repulsion between the interacting diene and dienophile and not by lowering the energy of the dienophile's LUMO and consequently, enhancing the normal electron demand orbital interaction. The Lewis acid binds via a donor-acceptor interaction to the dienophile and via that mechanism polarizes occupied orbital density away from the reactive C=C double bond of the dienophile towards the Lewis acid. This reduced occupied orbital density on C=C double bond of the dienophile will, in turn, engage in a less repulsive closed-shell-closed-shell orbital interaction with the incoming diene, reducing the destabilizing steric Pauli repulsion and hence lowers the Diels–Alder reaction barrier. In addition, the Lewis acid catalyst also increases the asynchronicity of the Diels–Alder reaction, making the occupied π-orbital located on the C=C double bond of the dienophile asymmetric. As a result, this enhanced asynchronicity leads to an extra reduction of the destabilizing steric Pauli repulsion as well as a diminishing pressure on the reactants to deform, in other words, it reduced the destabilizing activation strain (also known as distortion energy).[43] This working catalytic mechanism is known as Pauli-lowering catalysis,[44] which is operative in a variety of organic reactions.[45][46][47]
The original rationale behind Lewis acid-catalyzed Diels–Alder reactions is incorrect,[39][48][49][50] because besides lowering the energy of the dienophile's LUMO, the Lewis acid also lowers the energy of the HOMO of the dienophile and hence increases the inverse electron demand LUMO-HOMO orbital energy gap. Thus, indeed Lewis acid catalysts strengthen the normal electron demand orbital interaction by lowering the LUMO of the dienophile, but, they simultaneously weaken the inverse electron demand orbital interaction by also lowering the energy of the dienophile's HOMO. These two counteracting phenomena effectively cancel each other, resulting in nearly unchanged orbital interactions when compared to the corresponding uncatalyzed Diels–Alder reactions and making this not the active mechanism behind Lewis acid-catalyzed Diels–Alder reactions.
Asymmetric Diels–Alder
Many methods have been developed for influencing the stereoselectivity of the Diels–Alder reaction, such as the use of chiral auxiliaries, catalysis by
and many other methodologies exist for effecting diastereo- and enantioselective Diels–Alder reactions.Hexadehydro Diels–Alder
In the
Applications and natural occurrence

The retro-Diels–Alder reaction is used in the industrial production of cyclopentadiene. Cyclopentadiene is a precursor to various norbornenes, which are common monomers. The Diels–Alder reaction is also employed in the production of vitamin B6.
History

The work by Diels and Alder is described in a series of 28 articles published in the
Applications in total synthesis
The Diels–Alder reaction was one step in an early preparation of the steroids cortisone and cholesterol.[64] The reaction involved the addition of butadiene to a quinone.

Diels–Alder reactions were used in the original synthesis of prostaglandins F2α and E2.[65] The Diels–Alder reaction establishes the relative stereochemistry of three contiguous stereocenters on the prostaglandin cyclopentane core. Activation by Lewis acidic cupric tetrafluoroborate was required.

A Diels–Alder reaction was used in the synthesis of
.A synthesis of

In another synthesis of reserpine, the cis-fused D and E rings was formed by a Diels–Alder reaction. Intramolecular Diels–Alder of the

A pyranone was similarly used as the dienophile in the

A Diels–Alder reaction is a key step in the synthesis of (-)-furaquinocin C.[71]


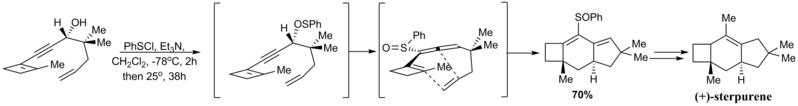
(+)-Sterpurene can be prepared by asymmetric D-A reaction
The tetracyclic core of the antibiotic (-)-tetracycline was prepared with a Diels–Alder reaction. Thermally initiated, conrotatory opening of the benzocyclobutene generated the o-quinodimethane, which reacted intermolecularly to give the tetracycline skeleton. The dienophile's free hydroxyl group is integral to the success of the reaction, as hydroxyl-protected variants did not react under several different reaction conditions.[74]

Takemura et al. synthesized cantharidin in 1980 by Diels–Alder reaction, utilizing high pressure.[75]
Synthetic applications of the Diels–Alder reaction have been reviewed extensively.[76][77][78][79][80]
See also
- Bradsher cycloaddition
- Wagner-Jauregg reaction
- Imine Diels–Alder reaction
- Aza-Diels–Alder reaction
References
- ISBN 978-0471264187.
- ISBN 978-0471264187.
- ^ PMID 19750686.
- .
- ISBN 978-0-7167-7266-8.
- ^ a b c d e f g Carey, Part B., pp. 474–526
- ^ PMID 22175326.
- ^ a b c Carey, Part A., pp. 836–50
- ^ a b Carey, Part A., p. 839
- .
- PMID 22175504.
- .
- .
- ^ .
- .
- .
- .
- ISBN 978-0471018193.
- .
- ^ Bérubé, G.; DesLongchamps, P. (1987). "Stéréosélection acyclique-1,5: Synthèse de la chaîne latérale optiquement active de la vitamine E". Bulletin de la Société Chimique de France. 1: 103–115.
- .
- .
- .
- )
- S2CID 26096085.
- ^ Carey, Part A, p. 149
- ^ .
- .
- .
- .
- .
- ^ Margareta Avram (1983). Chimie organica p. 318-323. Editura Academiei Republicii Socialiste România
- S2CID 260335918.
- ISBN 978-0-08-052349-1.
- .
- ISSN 0001-4842.
- ISBN 9780470746592.
- ISBN 9780199270293.
- ^ PMID 31944503.
- PMID 34094173.
- PMID 33169912.
- PMID 33780068.
- PMID 34499069.
- S2CID 232337915.
- PMID 32012430.
- PMID 33538169.
- S2CID 239089361.
- PMID 34094173.
- PMID 33169912.
- PMID 33780068.
- PMID 21462988.
- .
- .
- PMID 11942799.
- PMID 12785777.
- PMID 10891050.
- .
- PMC 3538845.
- PMC 8008985.
- S2CID 30482282.
- ISBN 978-3527306732.
- ^
- Diels, O.; Alder, K. (1928). "Synthesen in der hydroaromatischen Reihe, I. Mitteilung: Anlagerungen von "Di-en"-kohlenwasserstoffen". Justus Liebigs Annalen der Chemie. 460: 98–122. .
- Diels, O.; Alder, K. (1929). "Synthesen in der hydroaromatischen Reihe, II. Mitteilung: Über Cantharidin". Berichte der Deutschen Chemischen Gesellschaft. 62 (3): 554–562. .
- Diels, O.; Alder, K. (1929). "Synthesen in der hydroaromatischen Reihe, III. Mitteilung: Synthese von Terpenen, Camphern, hydroaromatischen und heterocyclischen Systemen. Mitbearbeitet von den Herren Wolfgang Lübbert, Erich Naujoks, Franz Querberitz, Karl Röhl, Harro Segeberg". Justus Liebigs Annalen der Chemie. 470: 62–103. .
- Diels, O.; Alder, K. (1929). "Synthesen in der hydroaromatischen Reihe, IV. Mitteilung: Über die Anlagerung von Maleinsäure-anhydrid an arylierte Diene, Triene und Fulvene (Mitbearbeitet von Paul Pries)". Berichte der Deutschen Chemischen Gesellschaft. 62 (8): 2081–2087. .
- Diels, O.; Alder, K. (1929). "Synthesen in der hydroaromatischen Reihe, V. Über Δ4-Tetrahydro-o-phthalsäure (Stellungnahme zu der Mitteilung von E. H. Farmer und F. L. Warren: Eigenschaften konjugierter Doppelbindungen (VII)". Berichte der Deutschen Chemischen Gesellschaft. 62 (8): 2087–2090. .
- Diels, O.; Alder, K. (1929). "Synthesen in der hydroaromatischen Reihe, VI. Mitteilung, Kurt Alder und Gerhard Stein: Über partiell hydrierte Naphtho- und Anthrachinone mit Wasserstoff in γ- bzw. δ-Stellung. (Mitbearbeitet von Paul Pries und Hans Winckler)". Berichte der Deutschen Chemischen Gesellschaft. 62 (8): 2337–2372. .
- Diels, O.; Alder, K. (1930). "Synthesen in der hydroaromatischen Reihe, VII. Mitteilung. (Mitbearbeitet von den Harren Ernst Petersen und Franz Querberitz.)". Justus Liebigs Annalen der Chemie. 478: 137–154. .
- Diels, O.; Alder, K. (1931). "Synthesen in der hydroaromatischen Reihe, VIII. Mitteilung: Dien-Synthesen des Anthracens. Anthracen-Forme". Justus Liebigs Annalen der Chemie. 486: 191–202. .
- Diels, O.; Alder, K. (1931). "Synthesen in der hydroaromatischen Reihe, IX. Mitteilung: Synthese des Camphenilons und des Santens". Justus Liebigs Annalen der Chemie. 486: 202–210. .
- Diels, O.; Alder, K. (1931). "Synthesen in der hydroaromatischen Reihe, X. Mitteilung: "Dien-Synthesen"︁ mit Pyrrol und seinen Homologen". Justus Liebigs Annalen der Chemie. 486: 211–225. .
- Diels, O.; Alder, K. (1931). "Synthesen in der hydroaromatischen Reihe, XI. Mitteilung. ("Dien-Synthesen"︁ des Cyclopentadiens, Cyclo-hexadiens und Butadiens mit Acetylen-dicarbonsäure und ihren Estern". Justus Liebigs Annalen der Chemie. 490: 236–242. .
- Diels, O.; Alder, K. (1931). "Synthesen in der hydroaromatischen Reihe, XII. Mitteilung. ("Dien-Synthesen"︁ sauerstoffhaltiger Heteroringe. 2. Dien-Synthesen des Furans.)". Justus Liebigs Annalen der Chemie. 490: 243–257. .
- Diels, O.; Alder, K. (1931). "Synthesen in der hydroaromatischen Reihe, XIII. Mitteilung. ("Dien-Synthesen"︁ sauerstoffhaltiger Heteroringe. 3. Dien-Synthesen der Cumaline.)". Justus Liebigs Annalen der Chemie. 490: 257–266. .
- Diels, O.; Alder, K. (1931). "Synthesen in der hydroaromatischen Reihe, XIV. Mitteilung. ("Dien-Synthesen"︁ stickstoffhaltiger Heteroringe. 2. Dien-Synthesen der Pyrrole mit Acetylen-dicarbonsäure und mit ihren Estern.)". Justus Liebigs Annalen der Chemie. 490: 267–276. .
- Diels, O.; Alder, K. (1931). "Synthesen in der hydroaromatischen Reihe, XV. Mitteilung. ("Dien-Synthesen"︁ stickstoffhaltiger Heteroringe. 3. Dien-Synthesen der Indole.)". Justus Liebigs Annalen der Chemie. 490: 277–294. .
- Diels, O.; Alder, K. (1932). "Synthesen in der hydroaromatischen Reihe, XVI. Mitteilung. ("Dien-Synthesen"︁ stickstoffhaltiger Heteroringe. 4. Dien-Synthesen der Pyrrole, Imidazole und Pyrazole.)". Justus Liebigs Annalen der Chemie. 498: 1–15. .
- Diels, O.; Alder, K. (1932). "Synthesen in der hydroaromatischen Reihe, XVII. Mitteilung. ("Dien-Synthesen"︁ stickstoffhaltiger Heteroringe. 5. Dien-Synthesen des Pyridins, Chinolins, Chinaldins und Isochinolins.)". Justus Liebigs Annalen der Chemie. 498: 16–49. .
- Diels, O.; Alder, K. (1933). "Synthesen in der hydroaromatischen Reihe, XVIII "Dien-Synthesen"︁ stickstoffhaltiger Heteroringe. 6. Dien-Synthesen des Pyridins. Zur Kenntnis des Chinolizins, Indolizins, Norlupinans und Pseudolupinins". Justus Liebigs Annalen der Chemie. 505: 103–150. .
- Diels, O.; Alder, K. (1934). "Synthesen in der hydroaromatischen Reihe, XIX. "Dien-Synthesen"︁ stickstoffhaltiger Heteroringe. 7. Zur Kenntnis der Primärprodukte bei den Dien-Synthesen des Pyridins, Chinolins und Chinaldins". Justus Liebigs Annalen der Chemie. 510: 87–128. .
- Diels, O.; Reese, J. (1934). "Synthesen in der hydroaromatischen Reihe, XX. Über die Anlagerung von Acetylen-dicarbonsäureester an Hydrazobenzol". Justus Liebigs Annalen der Chemie. 511: 168–182. .
- Diels, O.; Meyer, R. (1934). "Synthesen in der hydroaromatischen Reihe, XXI. "Dien-Synthesen"︁ stickstoffhaltiger Heteroringe. 8. Über den Verlauf der Dien-Synthese des Pyridins in methylalkoholischer Lösung". Justus Liebigs Annalen der Chemie. 513: 129–145. .
- Diels, O.; Friedrichsen, W. (1934). "Synthesen in der hydroaromatischen Reihe, XXII. Über die Anthracen–C4O3-Addukte, ihre Eignung zu Dien-Synthesen und ein neues Prinzip zur Synthese von Phtalsäuren und Dihydro-phtalsäuren". Justus Liebigs Annalen der Chemie. 513: 145–155. .
- Diels, O.; Möller, F. (1935). "Synthesen in der hydroaromatischen Reihe, XXIII. "Dien-Synthesen"︁ stickstoffhaltiger Heteroringe. 9. Stilbazol und Acetylen-dicarbonester". Justus Liebigs Annalen der Chemie. 516: 45–61. .
- Diels, O.; Kech, H. (1935). "Synthesen in der hydroaromatischen Reihe, XXIV "Dien-Synthesen"︁ stickstoffhaltiger Heteroringe". Justus Liebigs Annalen der Chemie. 519: 140–146. .
- Diels, O.; Reese, J. (1935). "Synthesen in der hydroaromatischen Reihe, XXV Über die Addukte aus Acetylen-dicarbonsäureester und Hydrazo-Verbindungen (2)". Justus Liebigs Annalen der Chemie. 519: 147–157. .
- Diels, O.; Harms, J. (1935). "Synthesen in der hydroaromatischen Reihe, XXVI. "Dien-Synthesen"︁ stickstoffhaltiger Heteroringe. 11. Über die aus Isochinolin und Acetylen-dicarbonsäureester entstehenden Addukte". Justus Liebigs Annalen der Chemie. 525: 73–94. .
- Diels, O.; Schrum, H. (1937). "Synthesen in der hydroaromatischen Reihe,XXVII. "Dien-Synthesen"︁ stickstoffhaltiger Heteroringe. 12. Über den Abbau der "gelben Substanz"︁ zu einem Isomeren des Norlupinans (1-Methyl-octahydro-indolizin)". Justus Liebigs Annalen der Chemie. 530: 68–86. .
- Diels, O.; Pistor, H. (1937). "Synthesen in der hydroaromatischen Reihe, XXVIII. "Dien-Synthesen"︁ stickstoffhaltiger Heteroringe. 13. α-Picolin und Acetylen-dicarbonsäureeste". Justus Liebigs Annalen der Chemie. 530: 87–98. .
- ^
"The Nobel Prize in Chemistry 1950". The Nobel Foundation. Retrieved 19 February 2016.
- .
- PMID 5808505.
- .
- .
- .
- S2CID 4371975.
- .
- .
- .
- .
- PMID 15941256.
- .
- ISBN 978-0471264187.
- ISBN 978-0471264187.
- ISBN 978-0471264187.
- ISBN 978-0471264187.
- ISBN 978-0471264187.
Bibliography
- Carey, Francis A.; Sundberg, Richard J. (2007). Advanced Organic Chemistry: Part B: Reactions and Synthesis (5th ed.). New York: Springer. ISBN 978-0387683546.
External links
- [1] English Translation of Diels and Alder's seminal 1928 German article that won them the Nobel prize. English title: 'Syntheses of the hydroaromatic series'; German title "Synthesen in der hydroaromatischen Reihe".
