HMX
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
1,3,5,7-Tetranitro-1,3,5,7-tetrazocane | |
| Other names
Octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine
| |
| Identifiers | |
3D model (
JSmol ) |
|
| ChEBI | |
| ChemSpider | |
ECHA InfoCard
|
100.018.418 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H8N8O8 | |
| Molar mass | 296.155 g/mol |
| Density | 1.91 g/cm3, solid |
| Melting point | 276 to 286 °C (529 to 547 °F; 549 to 559 K) |
| Explosive data | |
| Shock sensitivity | Low |
| Friction sensitivity | Low |
| Detonation velocity | 9100 m/s |
RE factor
|
1.70 |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Explosive |
| GHS labelling: | |
 
| |
| Danger | |
| H201, H205, H241, H301, H304, H311, H319 | |
| P210, P250, P280, P370+P380, P372, P373 | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
HMX, also called octogen, is a powerful and relatively insensitive
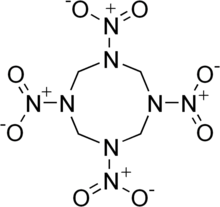
The molecular structure of HMX consists of an eight-membered ring of alternating carbon and nitrogen atoms, with a nitro group attached to each nitrogen atom. Because of its high mass-specific
Synthesis
HMX is more complicated to manufacture than most explosives, and this confines it to specialist applications. It and
Applications
Also known as cyclotetramethylene-tetranitramine, tetrahexamine tetranitramine, or octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine, HMX was first made in 1930. In 1949 it was discovered that HMX can be prepared by
HMX is used in melt-castable explosives when mixed with
HMX is also used in the process of perforating the steel casing in oil and gas wells. The HMX is built into a shaped charge that is detonated within the wellbore to punch a hole through the steel casing and surrounding cement out into the hydrocarbon-bearing formations. The pathway that is created allows formation fluids to flow into the wellbore and onward to the surface.[4][5]
The Hayabusa2 space probe used HMX to excavate a hole in an asteroid in order to access material that had not been exposed to the solar wind.[6]
Ongoing research aims to reduce its sensitivity and improve some manufacturing properties.[7][8]
Health and environmental fate
Analytical methods
HMX enters the environment through air, water, and soil because it is widely used in military and civil applications. At present, reverse-phase HPLC and more sensitive LC-MS methods have been developed to accurately quantify the concentration of HMX in a variety of matrices in environmental assessments.[9][10]
Toxicity
At present, the information needed to determine if HMX causes cancer is insufficient. Due to the lack of information, EPA has determined that HMX is not classifiable as to its human carcinogenicity.[11]
The available data on the effects on human health of exposure to HMX are limited. HMX causes CNS effects similar to those of RDX, but at considerably higher doses. In one study, volunteers submitted to patch testing, which produced skin irritation. Another study of a cohort of 93 workers at an ammunition plant found no hematological, hepatic, autoimmune, or renal diseases. However, the study did not quantify the levels of exposure to HMX.
HMX exposure has been investigated in several studies on animals. Overall, the toxicity appears to be quite low. HMX is poorly absorbed by ingestion. When applied to the dermis, it induces mild skin irritation but not delayed contact sensitization. Various acute and subchronic neurobehavioral effects have been reported in rabbits and rodents, including ataxia, sedation, hyperkinesia, and convulsions. The chronic effects of HMX that have been documented through animal studies include decreased hemoglobin, increased serum alkaline phosphatase, and decreased albumin. Pathological changes were also observed in the animals' livers and kidneys.
Gas exchange rate was used as an indicator of chemical stress in Northern bobwhite quail (Colinus virginianus) eggs, and no evidence of alterations in metabolic rates associated with HMX exposure was observed.
Biodegradation
Both wild and transgenic plants can phytoremediate explosives from soil and water.[16]
See also
- 2,4,6-Tris(trinitromethyl)-1,3,5-triazine
- 4,4’-Dinitro-3,3’-diazenofuroxan(DDF)
- Heptanitrocubane (HNC)
- HHTDD
- Octanitrocubane (ONC)
- RE factor
Notes
- ISBN 0-471-18636-8
- ^ a b John Pike (1996-06-19). "Nitramine Explosives". Globalsecurity.org. Retrieved 2012-05-24.
- ^ WE Bachmann, JC Sheehan (1949). "A New Method of Preparing the High Explosive RDX1". Journal of the American Chemical Society, 1949 (5):1842–1845.
- ^ Hansen, Brad (11 March 2013), "Technical Presentation Session 3: Drilling and Completion Casing Perforating Overview" (PDF), Casing Perforation Overview, EPA's Study of Hydraulic Fracturing and Its Potential Impact on Drinking Water Resources, U.S. Environmental Protection Agency
- .
- .
- .
- S2CID 209473864.
- PMID 19071351.
- PMID 1679187.
- ^ "Octahydro-1,3,5,7-tetranitro-1,3,5,7-tetr... (HMX) (CASRN 2691-41-0) | IRIS | US EPA." EPA. Environmental Protection Agency, n.d. Web. 15 Nov. 2012.[1]
- PMID 18279915.
- ^ "Fact Sheets". Mmr-iagwsp.org. Retrieved 2012-05-24.
- ^ Daniels, J. I.; Knezovich, J. P. (December 1994). "Information Bridge: DOE Scientific and Technical Information - Sponsored by OSTI" (PDF). Osti.gov. Retrieved 2012-05-24.
- ^ Newell, Charles. "Treatment of RDX & HMX Plumes Using Mulch Biowalls." ESTCP Project ER-0426. 2008.
- PMID 22996005.
References
- Cooper, Paul W. (1996). Explosives Engineering. New York: Wiley-VCH. OCLC 34409473. Retrieved 9 June 2014.
- Urbanski, Tadeusz (1967). Chemistry and Technology of Explosives. Vol. III. Warszawa: Polish Scientific Publishers.

