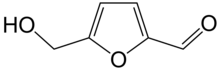
Hydroxymethylfurfural
| |

| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
5-(Hydroxymethyl)furan-2-carbaldehyde[1] | |
| Other names | |
| Identifiers | |
3D model (
JSmol ) |
|
| 110889 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
ECHA InfoCard
|
100.000.595 |
| EC Number |
|
| 278693 | |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H6O3 | |
| Molar mass | 126.111 g·mol−1 |
| Appearance | Low melting white solid |
| Odor | Buttery, caramel |
| Density | 1.29 g/cm3 |
| Melting point | 30 to 34 °C (86 to 93 °F; 303 to 307 K) |
| Boiling point | 114 to 116 °C (237 to 241 °F; 387 to 389 K) (1 mbar) |
| UV-vis (λmax) | 284 nm[2] |
| Related compounds | |
Related furan-2-carbaldehydes
|
Furfural |
| Hazards | |
| GHS labelling: | |
 [3] [3]
| |
| Warning[3] | |
| H315, H319, H335[3] | |
| P261, P305+P351+P338, P310[3] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Hydroxymethylfurfural (HMF), also known as 5-(hydroxymethyl)furfural, is an organic compound formed by the dehydration of reducing sugars.[4][5] It is a white low-melting solid (although commercial samples are often yellow) which is highly soluble in both water and organic solvents. The molecule consists of a furan ring, containing both aldehyde and alcohol functional groups.
HMF can form in sugar-containing food, particularly as a result of heating or cooking. Its formation has been the topic of significant study as HMF was regarded as being potentially carcinogenic to humans. However, so far in vivo genotoxicity was negative. No relevance for humans concerning carcinogenic and genotoxic effects can be derived.
Production and reactions
HMF was first reported in 1875 as an intermediate in the formation of
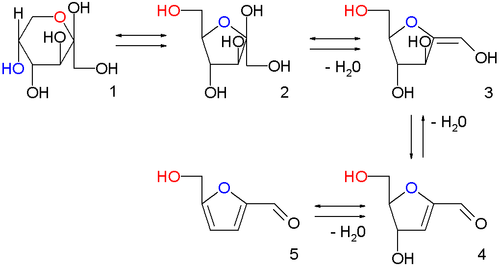
The classical approach tends to suffer from poor yields as HMF continues to react in aqueous acid, forming
Derivatives
HMF itself has few applications. It can however be converted into other more useful compounds.
Occurrence in food
HMF is practically absent in fresh food, but it is naturally generated in sugar-containing food during heat-treatments like drying or cooking. Along with many other flavor- and color-related substances, HMF is formed in the Maillard reaction as well as during caramelization. In these foods it is also slowly generated during storage. Acid conditions favour generation of HMF.[22] HMF is a well known component of baked goods. Upon toasting bread, the amount increases from 14.8 (5 min.) to 2024.8 mg/kg (60 min).[5] It is also formed during coffee roasting, with up to 769 mg/kg.[23]
It is a good

HMF can be found in low amounts in
Higher quantities of HMF are found naturally in coffee and dried fruit. Several types of roasted coffee contained between 300 – 2900 mg/kg HMF.[29] Dried plums were found to contain up to 2200 mg/kg HMF. In dark beer 13.3 mg/kg were found,[30] bakery-products contained between 4.1 – 151 mg/kg HMF.[31]
It can be found in glucose syrup.
HMF can form in
Depending on production-technology and storage, levels in food vary considerably. To evaluate the contribution of a food to HMF intake, its consumption-pattern has to be considered. Coffee is the food that has a very high relevance in terms of levels of HMF and quantities consumed.
HMF is a natural component in heated food but usually present in low concentrations. The daily intake of HMF may underlie high variations due to individual consumption-patterns. It has been estimated that the intakes range between 4 mg - 30 mg per person per day, while an intake of up to 350 mg can result from, e.g., beverages made from dried plums.[6][32]
Biomedical
A major metabolite in humans is 5-hydroxymethyl-2-furoic acid (HMFA), also known as Sumiki's acid, which is excreted in urine.
HMF bind intracellular sickle hemoglobin (HbS). Preliminary in vivo studies using transgenic sickle mice showed that orally administered 5HMF inhibits the formation of sickled cells in the blood.[33] Under the development code Aes-103, HMF has been considered for the treatment of sickle cell disease.[34]
Quantification
Today,
Other
HMF is an intermediate in the titration of hexoses in the Molisch's test. In the related Bial's test for pentoses, the hydroxymethylfurfural from hexoses may give a muddy-brown or gray solution, but this is easily distinguishable from the green color of pentoses.
Acetoxymethyl furfural (AMF) is also bio-derived green platform chemicals as an alternative to HMF.[38]
References
- ^ ISBN 978-0-85404-182-4.
- ^ The Determination of HMF in Honey with an Evolution Array UV-Visible Spectrophotometer. Nicole Kreuziger Keppy and Michael W. Allen, Ph.D., Application note 51864, Thermo Fisher Scientific, Madison, WI, USA (article)
- ^ a b c d Sigma-Aldrich Co., 5-(Hydroxymethyl)furfural.
- ^ PMID 23394139.
- ^ ISSN 1463-9262.
- ^ PMID 21462333.
- ^ PubChem. "EU Food Improvement Agents - PubChem Data Source". pubchem.ncbi.nlm.nih.gov. Retrieved 2018-06-25.
- ^ Commission Implementing Regulation (EU) No 872/2012 of 1 October 2012 adopting the list of flavouring substances provided for by Regulation (EC) No 2232/96 of the European Parliament and of the Council, introducing it in Annex I to Regulation (EC) No 1334/2008 of the European Parliament and of the Council and repealing Commission Regulation (EC) No 1565/2000 and Commission Decision 1999/217/EC Text with EEA relevance, 2012-10-02, retrieved 2018-06-25
- ^ Pubchem. "5-(Hydroxymethyl)-2-furaldehyde". pubchem.ncbi.nlm.nih.gov. Retrieved 2018-06-25.
- ^ S2CID 100848139.
- ^ ISSN 1424-6376.
- ISSN 0075-4617.
- S2CID 38432592.
- .
- ISSN 0019-7866.
- PMID 20973468.
- hdl:10044/1/31478.
- ISSN 1463-9262.
- ISSN 1759-9954.
- .
- PMID 20005914.
- PMID 33038777.
- PMID 24444020.
- hdl:10400.14/7635.
- PMID 18870652.
- ^ PMID 20491475.
- PMID 29619623.
- PMID 16917810.
- PMID 18929614.
- .
- PMID 21462333.
- S2CID 22342114.
- ^ "Aes-103 for Sickle Cell Disease". National Center for Advancing Translational Sciences. 2015-03-18. Retrieved 2022-01-20.
- PMID 479072.
- PMID 10890522.
- .
- PMID 25619448.
