Intramolecular force
This article needs additional citations for verification. (October 2017) |
An intramolecular force (or primary forces) is any force that binds together the atoms making up a molecule or compound, not to be confused with intermolecular forces, which are the forces present between molecules.[1] The subtle difference in the name comes from the Latin roots of English with inter meaning between or among and intra meaning inside.[2] Chemical bonds are considered to be intramolecular forces which are often stronger than intermolecular forces present between non-bonding atoms or molecules.
Types
The classical model identifies three main types of chemical bonds — ionic, covalent, and metallic — distinguished by the degree of charge separation between participating atoms.[3] The characteristics of the bond formed can be predicted by the properties of constituent atoms, namely electronegativity. They differ in the magnitude of their bond enthalpies, a measure of bond strength, and thus affect the physical and chemical properties of compounds in different ways. % of ionic character is directly proportional difference in electronegitivity of bonded atom.[clarification needed]
Ionic bond

An
. Sodium would give an electron to chlorine, forming a positively charged sodium ion and a negatively charged chloride ion.Covalent bond
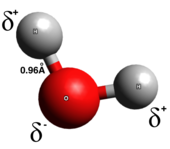
In a true
Metallic bond
Bond formation
Bonds are formed by atoms so that they are able to achieve a lower energy state. Free atoms will have more energy than a bonded atom. This is because some energy is released during bond formation, allowing the entire system to achieve a lower energy state. The bond length, or the minimum separating distance between two atoms participating in bond formation, is determined by their repulsive and attractive forces along the internuclear direction.[3] As the two atoms get closer and closer, the positively charged nuclei repel, creating a force that attempts to push the atoms apart. As the two atoms get further apart, attractive forces work to pull them back together. Thus an equilibrium bond length is achieved and is a good measure of bond stability.
Biochemistry

Intramolecular forces are extremely important in the field of biochemistry, where it comes into play at the most basic levels of biological structures. Intramolecular forces such as
See also
References
- OCLC 85824942.
- ^ "Inter vs. Intra". www.grammar.com. Retrieved 2018-04-26.
- ^ ISBN 978-0-8400-4931-5.
- .
- ^ "3.9: Intramolecular forces and intermolecular forces". Chemistry LibreTexts. 2022-04-05. Retrieved 2022-10-09.
- ^ Helmenstine, Anne Marie. "Understand What a Covalent Bond Is in Chemistry". ThoughtCo.
- OCLC 824794893.