Monomethyl auristatin E

| |
| Names | |
|---|---|
| IUPAC name
(S)-N-((3R,4S,5S)-1-((S)-2-((1R,2R)-3-(((1S,2R)-1-hydroxy-1-phenylpropan-2-yl)amino)-1-methoxy-2-methyl-3-oxopropyl)pyrrolidin-1-yl)-3-methoxy-5-methyl-1-oxoheptan-4-yl)-N,3-dimethyl-2-((S)-3-methyl-2-(methylamino)butanamido)butanamide
| |
| Other names
Monomethylauristatin E
| |
| Identifiers | |
3D model (
JSmol ) |
|
| Abbreviations | MMAE |
| ChemSpider | |
ECHA InfoCard
|
100.241.825 |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C39H67N5O7 | |
| Molar mass | 717.993 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Monomethyl auristatin E (MMAE) is a synthetic
antimitotic drug derived from peptides occurring in marine shell-less mollusc Dolabella auricularia called dolastatins which show potent activity in preclinical studies, both in vitro and in vivo, against a range of lymphomas, leukemia and solid tumors. These drugs show potency of up to 200 times that of vinblastine, another antimitotic drug used for Hodgkin lymphoma as well as other types of cancer.[2]
MMAE is actually desmethyl-auristatin E; that is, the N-terminal
methyl substituent instead of two as in auristatin E itself.[2]
Mechanism of action
Monomethyl auristatin E is an
antimitotic agent which inhibits cell division by blocking the polymerisation of tubulin. The linker to the monoclonal antibody is stable in extracellular fluid, but is cleaved by cathepsin once the conjugate has entered a tumor cell, thus activating the antimitotic mechanism.[3][4]
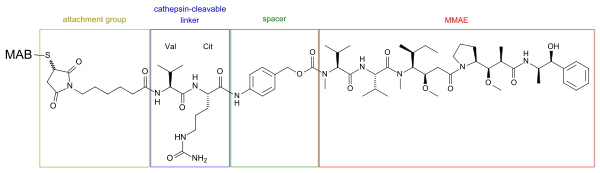
Structure of a MMAE-MAB-conjugate. The linker, consisting of the amino acids valine (Val) and citrulline (Cit), is cleaved by cathepsin inside tumour cells.[5] The spacer (para-aminobenzylcarbamate) is marked green, the cathepsin-cleavable linker is blue, and the attachment group (consisting of maleimide and caproic acid) is brown. The whole radical inside the four boxes is called vedotin.
Monoclonal antibodies/ADCs
MMAE has been tested with various monoclonal antibodies (usually forming an
antibody-drug conjugate
).
- targeting the protein Hodgkin's lymphoma:
- targeting the glycoprotein GPNMB which is found in aggressive melanoma, glioma, breast cancer and other tumours:
- targeting CD37:
- AGS67E, to treat
- targeting tissue factor in recurrent or persistent cervical cancers that progress on or after first line chemotherapy regimens:
Examples:
- Sofituzumab vedotin
- Polatuzumab vedotin (RG7596)
- Enfortumab vedotin
- Pinatuzumab vedotin
- Lifastuzumab vedotin
- Brentuximab vedotin
- Glembatumumab vedotin
- Tisotumab vedotin
- Indusatumab vedotin (MLN-0264) in phase II trials[11]
See also
- Seattle Genetics
- Monomethyl auristatin F
References
- ^ Statement on a nonproprietary name adopted by the USAN Council: Vedotin
- ^ PMID 22069744.
- ^ a b Seattle Genetics: Brentuximab vedotin (SGN-35) Archived 2011-07-16 at the Wayback Machine
- ^ S2CID 25746938.
- ^ A. Klement (13 May 2013). "Sprunginnovation beim Hodgkin-Lymphom: Adcetris". Österreichische Apothekerzeitung (in German) (10/2013): 67f.
- ^ Medical News Today: CuraGen Announces Expansion Of CR011-vcMMAE Phase II Trial In Advanced Breast Cancer
- ^ NCI Drug Dictionary: Glembatumumab vedotin
- PMID 25934707.
- ^ A study of Escalating Doses of AGS67E Given as Monotherapy in Subjects With Refractory or Relapsed Lymphoid Malignancies
- S2CID 233223114.
- ^ Indusatumab vedotin (MLN-0264) Clinical Trials. March 2015

