Temperature-responsive polymer
Temperature-responsive polymers or thermoresponsive polymers are polymers that exhibit drastic and discontinuous changes in their physical properties with temperature.[1] The term is commonly used when the property concerned is solubility in a given solvent, but it may also be used when other properties are affected. Thermoresponsive polymers belong to the class of stimuli-responsive materials, in contrast to temperature-sensitive (for short, thermosensitive) materials, which change their properties continuously with environmental conditions. In a stricter sense, thermoresponsive polymers display a miscibility gap in their temperature-composition diagram. Depending on whether the miscibility gap is found at high or low temperatures, either an upper critical solution temperature (UCST) or a lower critical solution temperature (LCST) exists.
Research mainly focuses on polymers that show thermoresponsivity in aqueous solution. Promising areas of application are tissue engineering,[2] liquid chromatography,[3][4] drug delivery[2][5] and bioseparation.[6] Only a few commercial applications exist, for example, cell culture plates coated with an LCST-polymer.
History
This section needs expansion. You can help by adding to it. (December 2012) |
The theory of thermoresponsive polymer (similarly, microgels) begins in the 1940s with work from Flory and Huggins who both independently produced similar theoretical expectations for polymer in solution with varying temperature.
The effects of external stimuli on particular polymers were investigated in the 1960s by Heskins and Guillet.[7] They established 32°C as the lower critical solution temperature (LCST) for poly(N-isopropylacrylamide).
Coil-globule transition
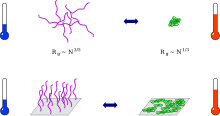
Thermoresponsive polymer chains in solution adopt an expanded coil conformation. At the phase separation temperature they collapse to form compact globuli. This process can be observed directly by methods of static and dynamic light scattering.[8][9] The drop in viscosity can be indirectly observed. When mechanisms which reduce surface tension are absent, the globules aggregate, subsequently causing turbidity and the formation of visible particles.
Phase diagrams of thermoresponsive polymers
The phase separation temperature (and hence, the cloud point) is dependent on polymer concentration. Therefore, temperature-composition diagrams are used to display thermoresponsive behavior over a wide range of concentrations.[10] Phases separate into a polymer-poor and a polymer-rich phase. In strictly binary mixtures the composition of the coexisting phases can be determined by drawing tie-lines. However, since polymers display a molar mass distribution this straightforward approach may be insufficient.
During the process of phase separation the polymer-rich phase can vitrify before equilibrium is reached. This depends on the
Thermodynamics
Polymers dissolve in a solvent when the
Without interactions between the compounds there would be no enthalpy of mixing and the entropy of mixing would be ideal. The ideal entropy of mixing of multiple pure compounds is always positive (the term -T∙ΔS is negative) and ΔG would be negative for all compositions, causing complete miscibility. Therefore, the fact that miscibility gaps are observed can only be explained by interaction. In the case of polymer solutions, polymer-polymer, solvent-solvent and polymer-solvent interactions have to be taken into account. A model for the phenomenological description of polymer phase diagrams was developed by Flory and Huggins (see Flory–Huggins solution theory). The resulting equation for the change of Gibbs energy consists of a term for the entropy of mixing for polymers and an interaction parameter that describes the sum of all interactions.[11]

where
- R = universal gas constant
- m = number of occupied lattice sites per molecule (for polymer solutions m1 is approximately equal to the degree of polymerization and m2=1)
- φ = volume fraction of the polymer and the solvent, respectively
- χ = interaction parameter
A consequence of the Flory-Huggins theory is, for instance, that the UCST (if it exists) increases and shifts into the solvent-rich region when the molar mass of the polymer increases. Whether a polymer shows LCST and/or UCST behavior can be derived from the temperature-dependence of the interaction parameter (see figure). It has to be noted that the interaction parameter not only comprises enthalpic contributions but also the non-ideal entropy of mixing, which again consists of many individual contributions (e.g., the strong hydrophobic effect in aqueous solutions). For these reasons, classical Flory-Huggins theory cannot provide much insight into the molecular origin of miscibility gaps.
Applications
Bioseparation
Thermoresponsive polymers can be functionalized with moieties that bind to specific biomolecules. The polymer-biomolecule conjugate can be precipitated from solution by a small change of temperature.[6][12] Isolation may be achieved by filtration or centrifugation.
Thermoresponsive surfaces
Tissue engineering
For some polymers it was demonstrated that thermoresponsive behavior can be transferred to surfaces. The surface is either coated with a polymer film or the polymer chains are bound covalently to the surface. This provides a way to control the wetting properties of a surface by small temperature changes. The described behavior can be exploited in
This way, it is possible to detach cells from a cell culture dish by only small changes in temperature, without the need to additionally use enzymes (see figure). Respective commercial products are already available.Chromatography
Thermoresponsive polymers can be used as the stationary phase in
Thermoresponsive gels
Covalently linked gels
Three-dimensional covalently linked polymer networks are insoluble in all solvents, they merely swell in good solvents.[16][17] Thermoresponsive polymer gels show a discontinuous change of the degree of swelling with temperature. At the volume phase transition temperature (VPTT) the degree of swelling changes drastically. Researchers try to exploit this behavior for temperature-induced drug delivery. In the swollen state, previously incorporated drugs are released easily by diffusion.[18] More sophisticated "catch and release" techniques have been elaborated in combination with lithography[19] and molecular imprinting.[20]
Physical gels
In physical gels unlike covalently linked gels the polymers chains are not covalently linked together. That means that the gel could re-dissolve in a good solvent under some conditions. Thermoresponsive physical gels, also sometimes called thermoresponsive injectable gels have been used in Tissue Engineering.[21][22][23][2][24] This involves mixing at room temperature the thermoresponsive polymer in solution with the cells and then inject the solution to the body. Due to the temperature increase (to body temperature) the polymer creates a physical gel. Within this physical gel the cells are encapsulated. Tailoring the temperature that the polymer solution gels can be challenging because this depend by many factors like the polymer composition,[25][26][27][28] architecture[25][26] as well as the molar mass.[27]
Thermoreversible materials
Some
Characterization of thermoresponsive polymer solutions
Cloud point
Experimentally, the phase separation can be followed by turbidimetry. There is no universal approach for determining the cloud point suitable for all systems. It is often defined as the temperature at the onset of cloudiness, the temperature at the inflection point of the transmittance curve, or the temperature at a defined transmittance (e.g., 50%).[11] The cloud point can be affected by many structural parameters of the polymer like the hydrophobic content,[25][26][27][28][34] architecture[25][26] and even the molar mass.[27][35]
Hysteresis
The cloud points upon cooling and heating of a thermoresponsive polymer solution do not coincide because the process of equilibration takes time. The temperature interval between the cloud points upon cooling and heating is called hysteresis. The cloud points are dependent on the cooling and heating rates, and hysteresis decreases with lower rates. There are indications that hysteresis is influenced by the temperature,
Other properties
Another important property for potential applications is the extent of phase separation, represented by the difference in polymer content in the two phases after phase separation. For most applications, phase separation in pure polymer and pure solvent would be desirable although it is practically impossible. The extent of phase separation in a given temperature interval depends on the particular polymer-solvent phase diagram.
Example: From the phase diagram of polystyrene (molar mass 43,600 g/mol) in the solvent cyclohexane it follows that at a total polymer concentration of 10%, cooling from 25 to 20 °C causes phase separation into a polymer-poor phase with 1% polymer and a polymer-rich phase with 30% polymer content.[37]
Also desirable for many applications is a sharp phase transition, which is reflected by a sudden drop in transmittance. The sharpness of the phase transition is related to the extent of phase separation but additionally relies on whether all present polymer chains exhibit the same cloud point. This depends on the polymer endgroups, dispersity, or—in the case of
Examples of thermoresponsive polymers
Thermoresponsivity in organic solvents
Due to the low entropy of mixing, miscibility gaps are often observed for polymer solutions.
Thermoresponsivity in water
Polymer solutions that show thermoresponsivity in water are especially important since water as a solvent is cheap, safe and biologically relevant. Current research efforts focus on water-based applications like drug delivery systems, tissue engineering, bioseparation (see the section
Some industrially relevant polymers show LCST as well as UCST behavior whereas the UCST is found outside the 0-to-100 °C region and can only be observed under extreme experimental conditions.
The UCST is dependent on the molecular mass of the polymer. For the LCST this is not necessarily the case, as shown for poly(N-isopropylacrylamide).[71][72][73]
Schizophrenic behavior of UCST-LCST diblock copolymers
A more complex scenario can be found in the case of diblock copolymers that feature two orthogonally thermo-responsive blocks, i.e., an UCST and an LCST-type block. By applying a temperature stimulus, the individual polymer blocks show different phase transitions, e.g. by increasing the temperature, the UCST-type block features an insoluble-soluble transition, while the LCST-type block undergoes a soluble-insoluble transition.[74][75][76] The order of the individual phase transitions depends on the relative positions of the UCST and LCST. Thus, upon temperature change the roles of the soluble and insoluble polymer blocks are reversed and this structural inversion is typically called ‘schizophrenic’ in the literature.[77][78][79] Besides the fundamental interest in the mechanism of this behavior, such block copolymers have been proposed for application in smart emulsification, drug delivery, and rheology control.[80][81][82] Schizophrenic diblock copolymer have also been applied as thin films for potential use as sensors, smart coatings or nanoswitches, and soft robotics.[83][84][85][86][59]
References
- PMID 7625927.
- ^ ISSN 2073-4360.
- ^ from the original on June 21, 2022. Retrieved October 4, 2022.
- ISSN 1466-8564.
- from the original on July 9, 2022. Retrieved October 4, 2022.
- ^ OCLC 54395177.
- .
- .
- .
- OCLC 45375807.
- ^ OCLC 756912488. Archived from the original (PDF) on 2022-08-10. Retrieved 2022-10-29. on Google Books. link to original publication.
- PMID 2090296.
- PMID 20358641.
- PMID 17623446.
- PMID 16830486.
- .
- ISBN 978-0-08-087862-1.
- ISSN 0168-3659.
- PMID 17441745.
- PMID 16530207.
- PMID 17507111.
- PMID 17881200.
- PMID 26614556.
- PMID 31355332.
- ^ (PDF) from the original on March 8, 2022. Retrieved June 19, 2021.
- ^ ISSN 1099-0518.
- ^ .
- ^ ISSN 1099-0518.
- PMID 33102942.
- ISBN 9781845698119, retrieved October 25, 2022
- ^ US application 2007202225, Chevalier, Olivier, "Cold gelling pastry glaze based on pectin", published 2007-08-30, assigned to Puratos NV, since abandoned.
- ^ Iso, Chef. "Neutral Nappage Glaze for Fruit Tarts". Chef Iso. Retrieved October 25, 2022.
- PMID 28092158.
- doi:10.1039/b926822g.
- ^ PMID 15530036.
- ^ PMID 22961764.
- ^ .
- S2CID 237539482.
- .
- .
- .
- .
- ISSN 0032-3861.
- .
- ISSN 0032-3861.
- ISSN 0300-9599.
- ISSN 0022-3654.
- ISSN 0022-233X.
- S2CID 95411474.
- .
- ISSN 0024-9297.
- PMID 32245164.
- ISSN 0022-3654.
- ISSN 0014-7672.
- ISSN 0032-3861.
- ISSN 0032-3861.
- ISSN 0024-9297.
- ISSN 0024-9297.
- ^ from the original on February 22, 2022. Retrieved February 24, 2022.
- PMID 29883371.
- PMID 17579398.
- PMID 23925439.
- ISSN 0014-3057.
- ISSN 0024-9297.
- .
- .
- ISSN 2046-2069.
- PMID 21928793.
- PMID 23610054.
- ISSN 0024-9297.
- ISSN 0024-9297.
- ISSN 1212-6950. Archived from the originalon October 29, 2022.
- ISSN 0022-2860.
- from the original on February 24, 2022. Retrieved February 24, 2022.
- from the original on February 24, 2022. Retrieved February 24, 2022.
- ISSN 0032-3861.
- PMID 28867829.
- ISSN 1381-5148.
- from the original on February 24, 2022. Retrieved February 24, 2022.
- PMID 30974657.
- from the original on February 24, 2022. Retrieved February 24, 2022.
- from the original on February 24, 2022. Retrieved February 24, 2022.
- from the original on February 24, 2022. Retrieved February 24, 2022.
- from the original on February 24, 2022. Retrieved February 24, 2022.
- from the original on February 24, 2022. Retrieved February 24, 2022.
- from the original on February 24, 2022. Retrieved February 24, 2022.


