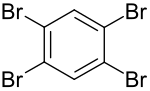
1,2,4,5-Tetrabromobenzene
| |
| Names | |
|---|---|
| Preferred IUPAC name
1,2,4,5-Tetrabromobenzene | |
| Identifiers | |
3D model (
JSmol ) |
|
ECHA InfoCard
|
100.010.231 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H2Br4 | |
| Appearance | white solid |
| Density | 2.518 g/cm3 |
| Melting point | 180–182 °C (356–360 °F; 453–455 K) |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H315, H319, H335, H413 | |
| P261, P264, P271, P273, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
1,2,4,5-Tetrabromobenzene is an
Preparation
The synthesis of 1,2,4,5-tetrabromobenzene has already been reported in 1865 from benzene and excess bromine in a sealed tube at 150 °C.[2] However, the clearly reduced melting point of about 160 °C indicates impurities in the final product. In his 1885 dissertation, Adolf Scheufelen published the synthesis of a purer sample using iron(III) chloride FeCl3 as a catalyst, isolated as "pretty needles" ("schönen Nadeln").[3]
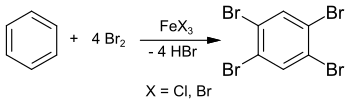
The synthesis can also be carried out in solution in chloroform or tetrachloromethane and yields 1,2,4,5-tetrabromobenzene in 89% yield.[4] This reaction can also be carried out in a laboratory experiment with excess bromine and iron nails (as starting material for iron (III) bromide FeBr3).[5] The intermediate stage is 1,4-dibromobenzene, which reacts further with excess bromine to give 1,2,4,5-tetrabromobenzene.
Reactions
Building block for liquid crystals and fluorescent dyes
Owing to its symmetrical structure and reactivity, 1,2,4,5-tetrabromobenzene is a precursor to
with an extensive planar, "board-like" tetrabenzoanthracene core.
In a one-pot reaction, 1,2,4,5-tetrabromobenzene reacts with
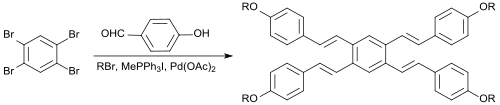
Due to their pronounced π-conjugation such compounds could be potentially applied as optical brighteners, OLED materials or liquid crystals.
N-alkyl-tetraaminobenzenes are available from 1,2,4,5-tetrabromobenzene in high yields, which can be cyclized with triethyl orthoformate and acids to benzobis(imidazolium) salts (BBI salts) and oxidized with oxygen to form 1,4-benzoquinone diimines.[10]
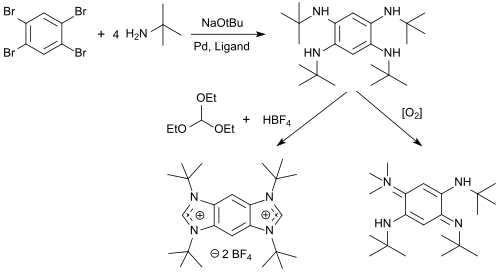
BBI salts are versatile
Starting material for arynes
From 1,2,4,5-tetrabromobenzene, a 1,4-monoarine can be prepared in-situ with one equivalent of n-butyllithium by bromine abstraction, which reacts immediately with furan to form 6,7-dibromo-1,4-epoxy-1,4-dihydronaphthalene (6,7-dibromonaphthalene-1,4-endoxide) in 70% yield.[12]

When 2,5-dialkylfurans (e.g. 2,5- (di-n-octyl)furan) are used, the dibrominated monoendoxide is formed in 64% yield, from which dibromo-5,8-di-n-octylnaphthalene is formed with zink powder/titanium tetrachloride in 88% yield.[13]

Upon treatment with titanium tetrachloride and zinc dust, the endoxide is deoxygenated yielding 2,3-dibromnaphthalene.[14]
The endoxide reacts with
If the dibromene oxide is allowed to react further with furan, in the presence of n-butyllithium
[2+4] cycloadditions with 1,2,4,5-tetrabromobenzene sometimes proceed in very high yields, such as the reaction of a dihalogen-substituted 1,3-diphenyl-isobenzofuran to a tetrahalogenated anthracene derivative (98%), which is converted successively further with 1,3-diphenyl isobenzofuran in 65% yield to a pentacene derivative and furan to a hexacene derivative (67%).[18]

The crosslinking of benzimidazole-modified polymers provides materials with a high absorption capacity for carbon dioxide, which could be suitable for CO2 separation from gas mixtures.[19]

It is the starting material for mono- and bis-aryines.[12]
Safety
1,2,4,5-Tetrabromobenzene is a liver toxic degradation product of the flame retardant hexabromobenzene and was already in 1987 detected in Japan in mother's milk samples.[20]
