Bdelloidea
| Bdelloid rotifers Temporal range:
| |
|---|---|

| |
| SEM showing morphological variation of bdelloid rotifers and their jaws | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Phylum: | Rotifera |
| Superclass: | Eurotatoria |
| Class: | Bdelloidea Hudson, 1884 |
Bdelloidea
Evolutionary relationships
The

Modern
The position of Bdelloidea within Syndermata (or Rotifera) is not entirely clear. Alternative possible phylogenetic relationships within the clade are illustrated by the accompanying cladograms. As of 2014, the "most comprehensive phylogenomic analysis of syndermatan relationships" to date was based on transcriptome data from all four groups,[8] and provided "strong support" for the hypothesis illustrated in the bottom left of the figure, in which Seisonidea and Acanthocephala are sister taxa. The study further indicated that the sister group to this taxon is Bdelloidea, whereas Monogononta is the outgroup to all three. This would mean that the closest living relatives of bdelloid rotifers are not monogonont rotifers, as previously believed, but seisonid rotifers and acanthocephalans, despite their highly modified morphology.
Classification and identification
Bdelloidea is a
Bdelloids can only be identified by eye while they are alive because many of the characteristics significant to classification are related to feeding and crawling; however, genetic identification of bdelloids is possible on dead individuals. Once preserved, the individuals contract into "blobs" which restricts analysis.[17] There are currently three morphological identification methodologies, two of which are considered dated: Bartoš (1951)[18] and Donner (1965).[14] The third method is a diagnostic key developed in 1995 by Shiel.[17]
Morphology
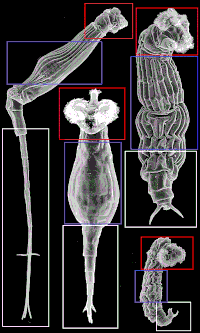
There are three main regions of the body of bdelloids: head, trunk and foot. The adjacent image depicts each area to show how body parts can be very different although they are named the same depending on the species involved. Bdelloids typically have a well-developed corona, divided into two parts, on a retractable head.
Some identifiable features of the bdelloids include :
- Well-developed foot glands[17]
- A mouth opening with a long oesophagus[17]
- Strong teeth (labelled by a tooth index)[17]
- Many cilia[17]
- Species-specific upper lip shape[17]
- Order-specific corona type[3]
- Philodinida consist of two ciliated discs
- Adinetida consist of a ventral ciliated field
- Philodinavida have a small corona
The bdelloid digestive and reproductive systems can be found within the trunk sections of their bodies, with the stomach being the most visible of the
Most bdelloids retract the foot while they eat, but there are four genera that lack a foot: Adineta, Bradyscela, Henoceros and Philodinavus. This affects not only how they feed but also how they crawl; for instance Adineta and Bradyscela slide whereas the other genera loop.[3]
Behaviour
The behaviour of bdelloids can be split into four categories: feeding, locomotion, reproduction and stress-induced behaviours.
Feeding
The specific feeding behaviour of bdelloids is varied but most use rings of cilia in the corona organ to create currents of water which blow food through the mouth to the mastax organ which has been adapted specifically for grinding food.[19] Food includes suspended bacteria, algae, detritus, and other things.
Locomotion
There appear to be three main methods of movement:
Reproduction
Bdelloids are of interest in the study of the
However, a new study provided evidence for interindividual genetic exchange and recombination in Adineta vaga, a species previously thought to be anciently asexual.[22]
Adineta vaga is capable of carrying out DNA repair by a nonreductional meiosis.[23] Germline DNA repair occurs in a specific period of oogenesis during which homologous chromosomes take on a meiotic-like juxtaposed configuration.[23] This germline DNA repair results in accurate reconstitution of the genetic material transmitted to offspring.
Evolution of obligate parthenogenetic reproduction
In 2003, the mode of asexual reproduction in the bdelloid rotifers was wholly unknown.[24] One theory of how obligate parthenogenesis arose in bdelloid rotifers was that parthenogenic lineages lost the ability to respond to sex-inducing signal, which is why these lineages retained their asexuality.[25] The obligate parthenogenetic strains of bdelloid rotifers produce a sex-inducing signal but have lost the ability to respond to that signal. It was later discovered that the inability to respond to sex-inducing signals in obligate parthenogens was caused by simple Mendelian inheritance of the gene op. [26]
Stress-induced behaviour
Bdelloids are able to survive environmental stresses by entering a state of dormancy known as
Bdelloidea have evolved a unique mechanism to help overcome one of the major perils of asexual reproduction. According to the Red Queen hypothesis of co-evolution, obligate asexuals will be driven extinct by rapidly changing parasites and pathogens, because they cannot change their genotypes quickly enough to keep up in this never-ending race. In populations of bdelloid rotifers, however, many parasites are destroyed during periods of extended desiccation.[31] Moreover, desiccated bdelloid rotifers are easily blown away from parasite-infested habitats by wind, and establish new, healthy populations elsewhere, which allows them to escape the Red Queen by moving in time and space instead of using sex to change their genotype.[32]
When these creatures recover from desiccation, it has been shown that they undergo a potentially unique genetic process where horizontal gene transfer occurs[citation needed], resulting in a significant proportion of the bdelloid genome, up to 10%, having been obtained through horizontal gene transfer from bacteria, fungi and plants.[33] How and why horizontal gene transfer occur in bdelloids is under much debate at present; particularly with regards to possible connections between the foreign genes and the desiccation process as well as possible connections to bdelloids' ancient asexuality.
When they desiccate completely, their DNA breaks up into many pieces. And when they come back to life after being rehydrated, it creates an opportunity for alien DNA fragments to enter their genome. This process was improved 60 million years ago when they captured a bacterial gene this way, which gave them a new gene regulatory system. The new system was used to keep transposons in check.[34]
Bdelloid rotifers are extraordinarily resistant to damage from ionizing radiation due to the same DNA-preserving adaptations used to survive dormancy.[35] These adaptations include an extremely efficient mechanism for repairing DNA double-strand breaks.[36] This repair mechanism was studied in two Bdelloidea species, Adineta vaga,[36] and Philodina roseola.[37] and appears to involve mitotic recombination between homologous DNA regions within each species.
Horizontal gene transfer
Large-scale horizontal transfer of bacterial, plant and fungal genes into bdelloid rotifers[38] has been documented, and may represent an important factor in bdelloid evolution.
Gallery
-
Lateral view of a bdelloid.
-
Frontal view of a bdelloid's corona.
-
Lateral view of a bdelloid.
-
Lateral view of a bdelloid.
-
Lateral view of a bdelloid in algae-rich water
-
Specimen of the genus Philodina
References
- .
- ISBN 978-1-86977-129-4.
- ^ S2CID 44054669.
- S2CID 13098228.
- ^ Renault, Marion (7 June 2021). "This Tiny Creature Survived 24,000 Years Frozen in Siberian Permafrost - The microscopic animals were frozen when woolly mammoths still roamed the planet, but were restored as though no time had passed". the New York Times. Retrieved 7 June 2021.
- S2CID 25975998.
- S2CID 20809514.
- ^ PMID 24520404.
- PMID 22927990.
- S2CID 28895228.
- ^ S2CID 24719056.
- ISBN 978-0199606030.
- S2CID 35937088.
- ^ ISBN 9789031908851.
- .
- PMID 19398026.
- ^ ISBN 978-0-646-22410-7.
- ^ Bartoš, Emanuel (1951). "The Czechoslovak Rotatoria of the order Bdelloidea". Mémoiresde la Société Zoologique Tchécoslovaque de Prague. 15: 241–500.
- S2CID 26501940.
- PMID 21237759.
- ^ Milius, Susan (1 November 2002). "Bdelloids: No sex for over 40 million years". Science News. Retrieved 6 November 2016.
- PMID 33339818.
- ^ PMID 36449626.
- .
- PMID 17995949.
- PMID 20862222.
- S2CID 84512992.
- ^ .
- .
- PMID 17767737.
- S2CID 43898914.
- PMID 23825214.
- PMID 21147969.
- ^ 60-M-Year-Old Bacteria Shed Light on 'New' DNA Modification System in Animals
- PMID 18362355.
- ^ PMID 25105197.
- PMID 18362354.
- S2CID 11862013.
External links
- Introduction to rotifers
- Rotifer World Catalog, by Jersabek C.D. & Leitner M.F.
- The Weird Sisters Archived 2015-10-01 at the Wayback Machine
- Bdelloids: No sex for over 40 million years Archived 2008-04-23 at the Wayback Machine
- An Evolutionary Scandal, from Harvard Magazine
- Who Needs Sex (or Males) Anyway?
- Tiny Creature Comes Back To Life After 24,000 Years In Siberian Deep Freeze Archived 2021-06-28 at the Wayback Machine






