Calcium propanoate
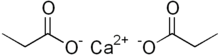
| |
| Names | |
|---|---|
| Preferred IUPAC name
Calcium dipropanoate | |
| Other names
Calcium propionate
Calcium dipropionate Mycoban | |
| Identifiers | |
3D model (
JSmol ) |
|
| ChemSpider | |
ECHA InfoCard
|
100.021.633 |
| EC Number |
|
| E number | E282 (preservatives) |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H10CaO4 | |
| Molar mass | 186.2192 g/mol |
| Appearance | White crystalline solid |
| Melting point | 300 °C |
| 49 g/100 mL (0 °C) 55.8 g/100 mL (100 °C) | |
| Solubility | slightly soluble in methanol, ethanol insoluble in acetone, benzene |
| Structure | |
| monoclinic | |
| Hazards | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Calcium propanoate or calcium propionate has the formula
Uses
As a food additive, it is listed as E number 282 in the Codex Alimentarius. Calcium propionate is used as a preservative in a wide variety of products, including: bread, other baked goods, processed meat, whey, and other dairy products.[2] In agriculture, it is used, amongst other things, to prevent milk fever in cows and as a feed supplement.[3] Propionates prevent microbes from producing the energy they need, like benzoates do. However, unlike benzoates, propionates do not require an acidic environment.[4]
Calcium propionate is used in bakery products as a mold inhibitor, typically at 0.1–0.4%[5] (though animal feed may contain up to 1%). Mold contamination is considered a serious problem amongst bakers, and conditions commonly found in baking present near-optimal conditions for mold growth.[6]
A few decades ago,
Metabolism of
Children were challenged with calcium propionate or placebo through daily bread in a double‐blind placebo‐controlled crossover trial. Although there was no significant difference by two measures, a statistically significant difference was found in the proportion of children whose behaviours "worsened" with challenge (52%), compared to the proportion whose behaviour "improved" with challenge (19%).
Calcium propionate can be used as a fungicide on fruit.[11]
In a 1973 study reported by the
In a recent well-designed translational study, human subjects fed 500 mg of calcium propionate twice daily demonstrated a modest decrease in LDL and total cholesterol, without a change in HDL. The study, only eight weeks in length, requires additional studies of both verification and longer duration to demonstrate the clinical value of this chemical. The study identified a novel regulatory circuit that links the gut microbiota metabolite propionic acid (PA), a short-chain fatty acid, with the gut immune system to control intestinal cholesterol homeostasis .[13]
References
- ^ Merck Index, 11th Edition, 1705.
- ^ Codex Alimentarius data for calcium propanoate Archived 2006-10-21 at the Wayback Machine
- ^ Center for Food and Nutrition Policy review of use of calcium propanoate as an organic agent in cow feed and as milk fever prevention
- ^ "Ingredients -- Calcium propionate". Retrieved 2007-03-10.
- ^ "NYSAES|FST|FVC|Venture 3| Chemical Food Preservatives". Archived from the original on 2010-04-12. Retrieved 2010-02-28.
- ^ "Keeping molds, bacteria at bay". Retrieved 2007-03-24.
- ^ Furia, T. E. (1973). CRC Handbook of Food Additives. CRC Handbook of Food Additives. CRC Press.
- ^ Furia, T. E. (1973). CRC Handbook of Food Additives. CRC Handbook of Food Additives. CRC Press.
- S2CID 24898218.
- S2CID 3054752.
- PMID 30861823.
- ^ "OPP PESTICIDE ECOTOXICITY DATABASE - Details - Pesticide: Calcium propionate". EPA / USDA / NIFA. Archived from the original on 2019-01-23. Retrieved 2019-01-22.
- PMC 9097250.

