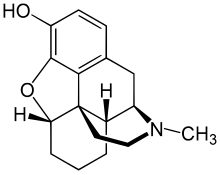
Desomorphine
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Permonid |
| Other names | Desomorphine, krokodil, dihydrodesoxymorphine, Permonid |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
JSmol) | |
| |
| |
| | |
Desomorphine
Desomorphine is a
A desomorphine product, usually based on codeine, has been developed as a recreational drug.[13] This is typically highly impure. The scaly sores and necrosis that develop around the injection site has prompted the name krokodil (Russian for crocodile).
Uses
Medical
Desomorphine was previously used in Switzerland for the treatment of severe pain. While medical usage of desomorphine was terminated in 1981, during the final years leading up to that it was being used to treat a single patient in
Recreational
Desomorphine abuse in
The drug can be made from
Adverse effects
Toxicity
Toxicity of desomorphine
Animal studies comparing pure desomorphine to morphine showed it to have increased toxicity, more potent relief of pain, higher levels of sedation, decreased respiration, and increased digestive activity.[25]
Toxicity of krokodil
Illicitly produced desomorphine is typically far from pure and often contains large amounts of
Causes of this damage are associated with
The frequent occurrence of tissue damage and infection among illicit users are what gained the drug its nickname of the
Reinforcement disorders
Abuse potential studies of desomorphine in animals had shown that it exhibited limited addiction liability. In monkeys, desomorphine had 10 times the depressant effect of morphine, developed tolerance less rapidly and less completely, and did not lead to the appearance of abstinence symptoms during withdrawal. Studies in rats receiving a daily injection of desomorphine at a constant dose showed that the animals developed tolerance slowly to the depressant effect of desomorphine. [25]
Chemistry

Desomorphine has a molecular weight of 271.35 g/mol and three salts are known: hydrobromide (as in the original Permonid brand; free-base conversion ratio 0.770), hydrochloride (0.881) and sulfate (0.802).[26] Its freebase form is slightly soluble in water (1.425 g/L at 25 °C), although its salts are very water-soluble; its freebase form is also very soluble in most polar organic solvents (like acetone, ethanol and ethyl acetate).[10] Its melting point is 189 °C.[10] It has a pKa of 9.69.[10] Desomorphine comes in four isoforms, A, B, C, and D[27] and the latter two appear to be the more researched and used.
Krokodil is made from codeine mixed with other substances. The codeine is retrieved from over-the-counter medicine and is then mixed with ethanol, gasoline, red phosphorus, iodine, hydrochloric acid and paint thinner.[10][28] Toxic nitrogen oxide fumes emerge from the drug when heated.[29]
History
It was first discovered and patented by a German team working for Knoll in 1920.[7] Desomorphine was later synthesised in the U.S. in 1932 and patented on 13 November 1934.[14] In Russia, desomorphine was declared an illegal narcotic analgesic in 1998. However, while codeine-containing drugs generally have been prescription products in Europe, in Russia they were sold freely over the counter until June 2012.[30] The number of users in Russia was estimated to have reached around one million at the peak of the drug's popularity.[31]
Society and culture
Legal status
In the US, desomorphine is a Schedule 1 controlled substance, indicating that the United States
North American media
Media in the U.S. and Canada have brought awareness to desomorphine. There have been incidents reported where desomorphine had supposedly been present within either country,[34] but no incidents have been confirmed by any drug testing or analytical results, and desomorphine use in North America is still considered unconfirmed.[35][36]
See also
- Conolidine
- Xylazine, a drug with similar side effects, widespread in the United States
Notes
References
- ^ Shuster S (5 December 2013). "The World's Deadliest Drug: Inside a Krokodil Cookhouse". Time.
- ^ Christensen J (18 October 2013). "Flesh-eating 'zombie' drug 'kills you from the inside out'". CNN.
- ISBN 978-0-306-42130-3.
- PMID 13546093.
- PMID 14451235.
- PMID 4098039.
- ^ a b DE Patent 414598C 'Verfahren zur Herstellung von Dihydrodesoxymorphin und Dihydrodesoxycodein'
- ^ a b US patent 1980972, Lyndon Frederick Small, "Morphine Derivative and Processes", published 1934-19-07, issued 1934-13-11
- ^ "Krokodil". New York State Office of Alcoholism and Substance Abuse Services. Archived from the original on 13 February 2014. Retrieved 27 September 2013.
- ^ PMID 24650492.
- .
- ^ Eddy NB, Howes HA (1935). "Studies of Morphine, Codeine and their Derivatives X. Desoxymorphine-C, Desoxycodeine-C and their Hydrogenated Derivatives". Journal of Pharmacology and Experimental Therapeutics. 55 (3): 257–67.
- S2CID 221476977.
- ^ S2CID 45615505.
- PMID 13511135.
- ^ Matiuk DM. Krokodil: A Monstrous Drug with Deadly Consequences. Journal of Addictive Disorders 2014; 1-14. Retrieved 17 April 2017 from Breining Institute at http://www.breining.edu
- S2CID 54546428.
- ^ PMID 23726898.
- PMID 25236385.
- PMID 22385107.
- ^ "Flesh-rotting 'krokodil' drug emerges in USA". USA Today.
- PMID 27391849.
- ^ Gogarty C (5 February 2019). "'Horrific' health problems of 'flesh-eating zombie drug' user". gloucestershirelive. Retrieved 6 February 2019.
- PMID 24173629.
- ^ a b c "DESOMORPHINE (Dihydrodesoxymorphine; dihydrodesoxymorphine-D; Street Name: Krokodil, Crocodil" (PDF). Drug Enforcement Administration. Archived from the original (PDF) on 11 January 2019. Retrieved 3 April 2014.
- ^ "Permonid". PubChem Compound. National Library of Medicine. Retrieved 5 May 2014.
- ISBN 9780412466304.
- PMID 25710781.
- ^ "Desomorphine". Specialized Information Services. Retrieved 3 April 2014.
- Gazeta.ru(in Russian). Retrieved 12 January 2014.
- ^ "Krokodil". New York State Office of Alcoholism and Substance Abuse Services. Archived from the original on 13 February 2014. Retrieved 3 April 2014.
- ^ "DEA Diversion Control Division". Archived from the original on 4 March 2016. Retrieved 4 May 2015.
- ^ "DEA Diversion Control Division". Archived from the original on 2 March 2016. Retrieved 4 May 2015.
- PMID 27222881.
- ISBN 978-1-77178-052-0.
- PMID 24970607.
External links
 Media related to Desomorphine at Wikimedia Commons
Media related to Desomorphine at Wikimedia Commons
