Perfluorotributylamine
| |
| |
| Names | |
|---|---|
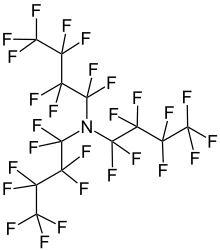
| Preferred IUPAC name
1,1,2,2,3,3,4,4,4-Nonafluoro-N,N-bis(nonafluorobutyl)butan-1-amine | |
| Other names
Fluorinert
| |
| Identifiers | |
3D model (
JSmol ) |
|
| Abbreviations | PFTBA |
| ChEBI | |
| ChemSpider | |
ECHA InfoCard
|
100.005.659 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| N(CF2CF2CF2CF3)3 | |
| Molar mass | 671.096 g·mol−1 |
| Appearance | colorless liquid |
| Density | 1.884 g/mL |
| Melting point | −50 °C (−58 °F; 223 K) |
| Boiling point | 178 °C (352 °F; 451 K) |
| Insoluble | |
| Solubility in methanol and isopropyl alcohol | Insoluble |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Perfluorotributylamine (PFTBA), also referred to as FC43, is an
Preparation
It is prepared by electrofluorination of tributylamine using hydrogen fluoride as solvent and source of fluorine:[2]
- N(CH2CH2CH2CH3)3 + 27 HF → N(CF2CF2CF2CF3)3 + 27 H2
Uses
The compound has two commercial uses. It is used as an ingredient in
Niche
The compound is used as a calibrant
Safety
Fluorofluids are generally of very low toxicity, so much that they have been evaluated as synthetic blood.[2]
Environmental impact
It is a greenhouse gas with warming properties more than 7,000 times that of carbon dioxide over a 100-year period,[5][6] and, as such, is one of the most potent greenhouse gasses ever discovered.[7] Its concentration in the atmosphere is approximately 0.18 parts per trillion. The compound can persist in the atmosphere for up to 500 years. Sulfur hexafluoride, however, has a GWP of 23,900,[8] which would make it much more powerful.
-
Global warming potential of greenhouse gases and PFTBA
See also
References
- ^ "Tuning basicity | Cambridge MedChem Consulting". www.cambridgemedchemconsulting.com. Retrieved 2020-08-11.
- ^ ISBN 978-0-471-23896-6.
- S2CID 38969204.
- ISBN 978-0-9882761-0-9, [1]., Nov. 2012.
- S2CID 130690897.
- ^ Goldenberg, Suzanne (10 December 2013). "Newly discovered greenhouse gas '7,000 times more powerful than CO2'". The Guardian. Retrieved 11 December 2013.
- ^ Goldenberg, Suzanne (11 December 2013). "Newly Discovered Greenhouse Gas "7,000 Times More Powerful than CO2"". Mother Jones. Retrieved 12 December 2013.
- ^ "2.10.2 Direct Global Warming Potentials". Intergovernmental Panel on Climate Change. 2007. Retrieved 22 February 2013.

