Podocyte
| Podocyte | |
|---|---|
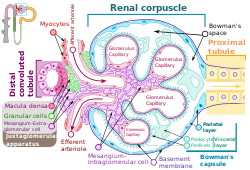 Renal corpuscle structure
Blood flows in the afferent arteriole at the top, and out the efferent arteriole at the bottom. Blood flows through the capillaries of the glomerulus, where it is filtered by pressure. The podocytes (green) are wrapped around the capillaries. Blood is filtered through the slit diaphragm (or filtration slit), between the feet or processes of the podocytes. The filtered blood passes out the proximal tubule (yellow) on the right. | |
| Details | |
| Precursor | Intermediate mesoderm |
| Location | Bowman's capsule of the kidney |
| Identifiers | |
| Latin | podocytus |
| MeSH | D050199 |
| FMA | 70967 |
| Anatomical terms of microanatomy | |
Podocytes are
The podocytes have long foot processes called pedicels, for which the cells are named (podo- + -cyte). The pedicels wrap around the capillaries and leave slits between them. Blood is filtered through these slits, each known as a filtration slit, slit diaphragm, or slit pore.[2] Several proteins are required for the pedicels to wrap around the capillaries and function. When infants are born with certain defects in these proteins, such as nephrin and CD2AP, their kidneys cannot function. People have variations in these proteins, and some variations may predispose them to kidney failure later in life. Nephrin is a zipper-like protein that forms the slit diaphragm, with spaces between the teeth of the zipper big enough to allow sugar and water through but too small to allow proteins through. Nephrin defects are responsible for congenital kidney failure. CD2AP regulates the podocyte cytoskeleton and stabilizes the slit diaphragm.[3][4]
Structure

Podocytes are found lining the Bowman's capsules in the nephrons of the kidney. The foot processes known as pedicels that extend from the podocytes wrap themselves around the

Podocytes secrete and maintain the basement membrane.[2]
There are numerous coated
Podocytes possess a well-developed
There is also growing evidence of a large number of multivesicular bodies and other lysosomal components seen in these cells, indicating a high endocytic activity.
Function

A. The endothelial cells of the glomerulus; 1. pore (fenestra).
B. Glomerular basement membrane: 1. lamina rara interna 2. lamina densa 3. lamina rara externa
C. Podocytes: 1. enzymatic and structural protein 2. filtration slit 3. diaphragma
Podocytes have primary processes called trabeculae, which wrap around the

Small molecules such as
.Podocytes are also involved in regulation of glomerular filtration rate (GFR). When podocytes contract, they cause closure of filtration slits. This decreases the GFR by reducing the surface area available for filtration.
Clinical significance

A loss of the foot processes of the podocytes (i.e., podocyte effacement) is a hallmark of minimal change disease, which has therefore sometimes been called foot process disease.[13]
Disruption of the filtration slits or destruction of the podocytes can lead to massive proteinuria, where large amounts of protein are lost from the blood.
An example of this occurs in the congenital disorder
In 2002 Professor Moin Saleem at the University of Bristol made the first conditionally immortalised human podocyte cell line.[14][further explanation needed] This meant that podocytes could be grown and studied in the lab. Since then many discoveries have been made. Nephrotic syndrome occurs when there is a breakdown of the glomerular filtration barrier. The podocytes form one layer of the filtration barrier. Genetic mutations can cause podocyte dysfunction leading to an inability of the filtration barrier to restrict urinary protein loss. There are currently 53 genes known to play a role in genetic nephrotic syndrome.[15] In idiopathic nephrotic syndrome, there is no known genetic mutation. It is thought to be caused by a hitherto unknown circulating permeability factor.[16] Recent evidence suggests that the factor could be released by T-cells or B-cells,[17][18] podocyte cell lines can be treated with plasma from patients with nephrotic syndrome to understand the specific responses of the podocyte to the circulating factor. There is growing evidence that the circulating factor could be signalling to the podocyte via the PAR-1 receptor.[19][further explanation needed]
Presence of podocytes in urine has been proposed as an early diagnostic marker for
See also
- List of human cell types derived from the germ layers
- List of distinct cell types in the adult human body
References
- ^ "Podocyte" at Dorland's Medical Dictionary
- ^ ISBN 978-1-4614-3784-0.
- S2CID 43237744.
- PMID 19562370.
- ^ Nosek TM. "Epithelium; Cell Types". Essentials of Human Physiology. Archived from the original on 24 March 2016.
- ^ ISBN 978-1-4557-0307-4. Retrieved 2 June 2020.
- PMID 19374010.
- PMID 15545998.
- PMID 20233749.
- PMID 19478094.
- PMID 12724416.
- license
- PMID 27940460.
- PMID 11856766.
- S2CID 4768411.
- PMID 25416821. Retrieved 26 April 2023.
- PMID 34792685.
- PMID 31339775.
- S2CID 257639270.
- PMID 23709369.
External links
- Anatomy photo: Urinary/mammal/vasc1/vasc1 - Comparative Organology at University of California, Davis - "Mammal, renal vasculature (EM, High)
- Histology image: 22401loa – Histology Learning System at Boston University - ". Ultrastructure of the Cell: podocytes and glomerular capillaries"
- UIUC Histology Subject 1400
- podocyte.ca[Samuel Lunenfeld Research Institute
- Histology image: 22402loa – Histology Learning System at Boston University
- Histology image: 22403loa – Histology Learning System at Boston University
