Tin(IV) bromide

| |
| |
| Names | |
|---|---|
| IUPAC name
tetrabromostannate
| |
| Other names
tin tetrabromide, stannic bromide, bromostannic acid
| |
| Identifiers | |
3D model (
JSmol ) |
|
| ChemSpider | |
ECHA InfoCard
|
100.029.258 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| SnBr4 | |
| Molar mass | 438.33 g/mol |
| Appearance | colourless [1] |
| Density | 3.340 g/cm3 (at 35 °C)[1] |
| Melting point | 31 °C (88 °F; 304 K)[1] |
| Boiling point | 205 °C (401 °F; 478 K)[1] |
| soluble | |
| −149.0·10−6 cm3/mol | |
| Related compounds | |
Other anions
|
Tin(IV) fluoride Tin(IV) chloride Tin(IV) iodide |
Other cations
|
Carbon tetrabromide Silicon tetrabromide Germanium tetrabromide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Tin(IV) bromide is the
low melting solid.[1]
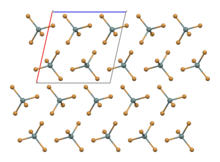
Structure
SnBr4 occurs in form of crystals. The compound crystallises in a monoclinic crystal system with molecular SnBr4 units that have distorted tetrahedral geometry,[2] with mean Sn-Br bond lengths of 242.3 pm.[3]
Preparation
SnBr4 can be prepared by reaction of the elements at standard temperature and pressure (STP):[4][page needed]
- Sn + 2Br
2 → SnBr
4
Dissolution in solvents
In aqueous solution Sn(H2O)64+[contradictory] is the principal ionic species amongst a range of 6 coordinate ions with from 0-6 bromide ligands (e.g. Sn(H2O)64+, SnBr(H2O)53+). In basic solution the Sn(OH)62− ion is present.[5]
Reactions
SnBr4 forms 1:1 and 1:2 complexes with ligands, e.g. with trimethylphosphine the following can be produced, SnBr4.P(CH3)3 and SnBr4.2P(CH3)3.[6]
References
- ^ ISBN 978-0-08-037941-8.
- .
- S2CID 94609783.
- OCLC 1024925228.
- .
- doi:10.1139/v73-406.
