Acyl halide
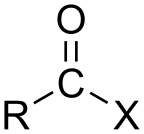
In
If the acid is a
Preparation
Aliphatic acyl halides
On an industrial scale, the reaction of acetic anhydride with hydrogen chloride produces a mixture of acetyl chloride and acetic acid:[5]
- (CH3CO)2O + HCl → CH3COCl + CH3CO2H
Common syntheses of acyl chlorides also entail the reaction of carboxylic acids with phosgene, thionyl chloride,[6] and phosphorus trichloride[7] Phosphorus pentabromide is used for acyl bromides, which are rarely of value.
Aromatic acyl chlorides
Benzoyl chloride is produced from benzotrichloride using either water or benzoic acid:[8]
- C6H5CCl3 + H2O → C6H5COCl + 2 HCl
- C6H5CCl3 + C6H5CO2H → 2 C6H5COCl + HCl
As with other acyl chlorides, it can be generated from the parent acid and other chlorinating agents phosphorus pentachloride or thionyl chloride.
Representative laboratory routes to
Acyl fluorides
Of commercial interest,
Carboxylic acids react with sulfur tetrafluoride to give the acyl fluoride:[13]
- SF4 + RCO2H → SOF2 + RC(O)F + HF
Acyl bromides and iodides
Acyl bromides and iodides are synthesized accordingly but are less common.[14]
Reactions
Acyl halides are rather reactive compounds often synthesized to be used as intermediates in the synthesis of other organic compounds. For example, an acyl halide can react with:
- water, to form a carboxylic acid. This hydrolysis is the most heavily exploited reaction for acyl halides as it occurs in the industrial synthesis of acetic acid.
- an Friedel-Crafts acylation.
- carboxylic acids to form an organic acid anhydrides.[15]
In the above reactions, HX (hydrogen halide or hydrohalic acid) is also formed. For example, if the acyl halide is an acyl chloride, HCl (hydrogen chloride or hydrochloric acid) is also formed.
Multiple functional groups

A molecule can have more than one acyl halide functional group. For example, "adipoyl dichloride", usually simply called adipoyl chloride, has two acyl chloride functional groups; see the structure at right. It is the dichloride (i.e., double chloride) of the 6-carbon dicarboxylic acid adipic acid. An important use of adipoyl chloride is polymerization with an organic di-amino compound to form a polyamide called nylon or polymerization with certain other organic compounds to form polyesters.
Phosgene (carbonyl dichloride, Cl–CO–Cl) is a very toxic gas that is the dichloride of carbonic acid (HO–CO–OH). Both chlorine atoms in phosgene can undergo reactions analogous to the preceding reactions of acyl halides. Phosgene is used a reactant in the production of polycarbonate polymers, among other industrial applications.
General hazards
Volatile acyl halides are
References
- ISBN 9780470771273.
- ISBN 3527306730.
- .
- ^ .
- ISBN 3527306730.
- .
- .
- .
- S2CID 73481495.
- ISBN 978-0-471-26418-7.
- ISSN 0022-3263.
- PMID 20280752.



