Endophyte
An endophyte is an
History
Endophytes were first described by the German botanist Johann Heinrich Friedrich Link in 1809. They were thought to be plant parasitic fungi and they were later termed as "microzymas" by the French scientist Béchamp. There was a belief that plants were healthy under sterile conditions and it was not until 1887 that Victor Galippe discovered bacteria normally occurring inside plant tissues.[2] Though, most of the endophytic studies reports the mutualistic relationship of bacteria and fungus, Das et al., (2019) reported about endophytic virome and their probable function in plant defense mechanisms.[3]
Transmission
Endophytes may be transmitted either vertically (directly from parent to offspring) or horizontally (among individuals).
Symbiosis
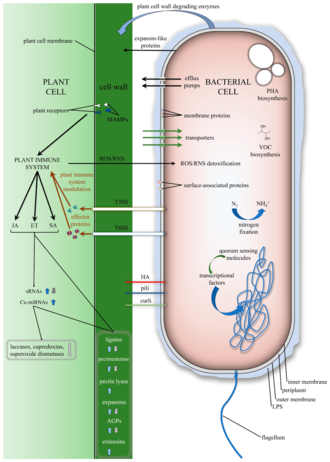
Most endophyte-plant relationships are still not well understood.[7] However, recently it was shown that endophytes are transmitted from one generation to another via seeds, in a process called vertical transmission.[8] Endophytes and plants often engage in mutualism, with endophytes primarily aiding in the health and survival of the host plant with issues such as pathogens and disease,[9] water stress, heat stress, nutrient availability and poor soil quality, salinity, and herbivory.[2] In exchange the endophyte receives carbon for energy from the plant host. Plant-microbe interactions are not strictly mutualistic, as endophytic fungi can potentially become pathogens or saprotrophs, usually when the plant is stressed.[10] Endophytes may become active and reproduce under specific environmental conditions or when their host plants are stressed or begin to senesce, thereby limiting the amount of carbon provided to the endophyte.[11][12]
Endophytes may benefit host plants by preventing other pathogenic or parasitic organisms from colonizing them. Endophytes can extensively colonize plant tissues and competitively exclude other potential pathogens.[13][14] Some fungal and bacterial endophytes have proven to increase plant growth and improve overall plant hardiness.[15]
Studies have shown that endophytic fungi grow in a very intimate interaction with their host plant cells. Fungal
The presence of certain fungal endophytes in host
There is evidence that plants and endophytes engage in communication with each other that can aid symbiosis. For example, plant chemical signals have been shown to activate gene expression in endophytes. One example of this plant-endosymbiont interaction occurs between
Effects on plant behavior
There are various behaviors that have been studied that resulted from endophyte symbiosis with plants. Through association with fungal endophytes, the root and shoot structures of Pseudotsuga menziesii (Douglas-fir) saplings in low-nutrient conditions have been shown to be elongated, as well as undergo overall biomass increases.[20] The proposed mechanisms behind this include high inorganic phosphate solubilization ability by the fungi as well as organic phosphate mineralization, increased mycorrhizal associations through root colonization, and enhanced nitrogen and phosphorus uptake.[20] Specific endophyte species can also stimulate root growth by increasing the flux of auxin to where the endophyte is.[21]
Additionally, various reports on endophyte interactions have shown increased photosynthetic capacities of host plants as well as improved water relations.[22] Improvements in water use efficiency were observed in higher CO2 concentrations and a further increase was seen in water deficit conditions.[22] In addition, other various physiological pathways were activated upon endophytes interactions with host plants, enabling tighter water control and further water management, which are to be the main reasons behind improved water relations.[22] Specifically, evidence points to endophytes producing ABA to affect stomatal conductance as well as microbial respiration and plants recycling CO2.[23]
However, the specific biochemical mechanisms behind these behavioral changes are still largely unknown and lower-level signal cascades have yet to be discovered. Furthermore, while the benefits of endophyte relations are well-studied, the costs of these relations are less well understood, such as the specific carbon costs, the system of endophyte governance, and the environmental conditions that facilitate a proper plant-endophyte relationship.[22]
In an experiment investigating the interaction between Miscanthus sinensis and the plant endophyte Herbaspirillum frisingense, a roughly 20% increase in fresh biomass was observed in M. sinensis following inoculation with H. frisingense.[24] However, unique to this experiment was the mode by which this was thought to happen. Inoculation saw an upregulation in the genes relevant to jasmonate and ethylene production in the plant roots, although the mechanism to this is still unknown.[24] Specifically, H. frisingense was shown to upregulate ethylene receptors and repress ethylene response factors, overall leading to an increase in root growth.[24] Additionally, H. frisingense is known to produce indoleacetic acid (IAA),[25] and was also shown to manage IAA genes, indicating that there is an intricate balance maintained between ethylene and IAA by H. frisingense.[24]
Diversity
Endophytic species are very diverse; only a small minority of existing endophytes have been characterized.[26][27] Many endophytes are in the phyla Basidiomycota and Ascomycota. Endophytic fungi may be from Hypocreales and Xylariales of the Sordariomycetes (Pyrenomycetes) class or from the class of Loculoascomycetes.[28] One group of fungal endophytes are the arbuscular mycorrhizal fungi involving biotrophic Glomeromycota associated with various plant species.[29] As often with other organisms associated with plants such as mycorrhizal fungus, endophytes gain carbon from their association with the plant host. Bacterial endophytes are polyphyletic, belonging to broad range of taxa, including α-Proteobacteria, β-Proteobacteria, γ-Proteobacteria, Firmicutes, Actinobacteria.[30]
One or more endophytic organisms are found in nearly every land plant.[31] It is suggested that areas of high plant diversity such as tropical rainforests may also contain the highest diversity of endophytic organisms that possess novel and diverse chemical metabolites.[32] It has been estimated that there could be approximately 1 million endophytic fungi that exist in the world.[32]
A diazotrophic bacterium isolated in lodgepole pines (Pinus contorta) in British Columbia, Canada, is Paenibacillus polymyxa, which may help its host by fixing nitrogen.[33][34][35][36][37][38]
Classification
Endophytes include a wide variety of microorganisms including fungi, bacteria and viruses. There are two different means of classifying endophytes.
Systemic and non-systemic
The first method divides endophytes into two categories: systemic (true) and nonsystemic (transient). These categories are based on the endophyte's genetics, biology, and mechanism of transmission from host to host.[39] Systemic endophytes are defined as organisms that live within plant tissues for the entirety of its life cycle and participate in a symbiotic relationship without causing disease or harm to the plant at any point. Additionally, systemic endophytes concentrations and diversity do not change in a host with changing environmental conditions.[39] Non-systemic or transient endophytes on the other hand vary in number and diversity within their plant hosts under changing environmental conditions. Non-systemic endophytes have also been shown to become pathogenic to their host plants under stressful or resource limited growing conditions.[39] An example of this would be Colletotrichum fioriniae, which is an endophyte of many temperate broadleaved trees and shrubs, but can also be a pathogen on many fruits and some leaves.[40][41]
Clavicipitaceous and non-clavicipitaceous
The second method divides fungal endophytes into four groups based on taxonomy and six other criteria: host range, host tissues colonized, in planta colonization, in planta biodiversity, mode of transmission and fitness benefits.[42] These four groups are divided into clavicipitaceous endophytes (Class 1) and non-clavicipitaceous endophytes (Class 2, 3, and 4).
Class 1 endophytes are all phylogenetically related and proliferate within cool and warm season grasses. They typically colonize plant shoots where they form a systemic intercellular infection. Class 1 endophytes are mainly transmitted from host to host by vertical transmission, in which maternal plants pass fungi on to their offspring through seeds. Class 1 endophytes can further be divided into Types I, II and III. Among these three types of clavicipitaceous endophytes are different interactions with their plant hosts. These interaction range from pathogenic to symbiotic and symptomatic to asymptomatic. Type III clavicipitaceous endophytes grow within their plant host without manifesting symptoms of disease or harming their host. Class 1 endophytes typically confer benefits on their plant host such as improving plant biomass, increasing drought tolerance and increasing the production of chemicals that are toxic and unappetizing to animals, thereby decreasing herbivory. These benefits can vary depending on the host and environmental conditions.[42]
Non-clavicipitaceous endophytes represent a
Applications
Endophytes may have potential future applications in agriculture.
Biofuel
A 2008 experiment with an isolate of a fungus called NRRL 50072 found that this strain can produce a small amount of fuel-like hydrocarbon compounds which was promoted as "myco-diesel". It was hoped that perhaps in the future this might provide a possible source of
A strain of endophytic fungi which appeared to be closely related to Nigrograna mackinnonii which was isolated from a stem of the plant Guazuma ulmifolia collected in Ecuador was found to produce a variety of volatile organic compounds including terpenes and odd chain polyenes. The polyenes isolated from the fungus have properties that are sought in gasoline-surrogate biofuels.[54]
Phytoremediation
Plants are potentially able to break down or sequester, or stimulate micro-organisms in the soil to break down or sequester, certain organic pollutants and inorganic pollutants such as
Two strains of the endophytic fungi Pestalotiopsis microspora isolated from stems of plants from the Ecuadorian rainforest were shown in laboratory experiments to be able to digest polyurethane plastic as the fungus's sole carbon source in anaerobic conditions, although many other non-endophytic fungi have demonstrated this ability, and most isolates of endophytic fungi in this experiment could perform this to some degree.[56]
Drug discovery
Endophytes produce a wide variety of
A well known example of the discovery of chemicals derived from endophytic fungi is from the fungus Taxomyces andreanae isolated from the pacific yew Taxus brevifolia. T. andreanae produces paclitaxel, also known as taxol. This drug is important for the treatment of cancer. Other endophytes since have been discovered that also produce paclitaxel in other host species, but to date there has been no successful industrial source of paclitaxel created.[62]
Endophytes have been discovered with various anti-tumor properties. Endophytic fungi produce many secondary compounds such as alkaloids, triterpenes and steroids which have been shown to have anti-tumor effects.[59] The alkaloid beauvericin has been isolated from the fungus Fusarium oxysporum and has shown cytotoxicity against the tumor cells PC3, PANC-1, and A549.[64][65] Two fusarubin derivatives: anhydrofusarubin and methyl ether of fusarubin were isolated from endophytic fungus Cladosporium sp. and have shown cytotoxicity against human leukemia (K-562).[59] Three triterpenes were found in the endophyte Xylarialean sp., all three of these compounds displayed mild cytotoxic effects on tumor cells.[65]
Some of the antimicrobial compounds produced by endophytic fungi are of interest in their effectiveness against pathogens which have developed resistances to antibiotics. Different fractions of
An endophytic fungus of the genus Pseudomassaria has been found in the rainforest of the Democratic Republic of the Congo. This fungus yields a metabolite that shows potential as an antidiabetic, also known as an insulin mimetic. This compound acts like insulin and has been shown to lower blood glucose levels in mouse model experiments.[31]
Agriculture
Among the many promising applications of endophytic microbes are those intended to increase agricultural use of endophytes to produce crops that grow faster and are more resistant and hardier than crops lacking endophytes.
Endophytes appear to enhance the growth of their plant host symbionts. Endophytes also provide their hosts with an increased resilience to both abiotic and biotic stressors such as drought, poor soils and herbivory. The increased growth and resilience is likely caused by the endophytes ability to improve plant nutrition or secondary metabolite production, as in the case of
Many endophytes protect plants from herbivory from both insects and animals by producing secondary metabolites that are either unappetizing or toxic to the herbivore.
There are several endophytes that have been discovered that exhibit insecticidal properties. One such endophyte comes from the Nodulisporium sp. which was first harvested from the plant
There are many obstacles to successfully implementing the use of endophytes in agriculture. Despite the many known benefits that endophytes may confer to their plant hosts,
See also
- Biofertilizer
- List of endophytes
- Plant use of endophytic fungi in defense
- Arbuscular mycorrhiza
- Mycorrhiza
- Rhizobia
References
- S2CID 252376815.
- ^ PMID 26136581.
- S2CID 146037776.
- .
- S2CID 24846604.
- PMID 31010043..
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License - PMID 25567944.
- PMID 33427409.
- S2CID 230508404.
- ISBN 9780986399602.
- ^ PMID 27375610.
- S2CID 37813335.
- .
- ISSN 2171-9292.
- PMID 18789693.
- ^ PMID 17919950.
- PMID 11722882.
- PMID 20955417.
- PMID 22840767.
- ^ doi:10.3390/f6103582.
- PMID 28348550.
- ^ ISBN 978-3-319-65897-1, retrieved 2021-06-10
- PMID 29552021.
- ^ PMID 24043849.
- S2CID 913558.
- ISBN 9788132226451.
- PMID 19236579.
- ISBN 978-94-007-1598-1.
- PMID 25639293.
- .
- ^ PMID 14665674.
- ^ PMID 25620957.
- S2CID 92275030.
- PMID 31647534.
- S2CID 92247486.
- .
- S2CID 213458305.
- PMID 32845898.
- ^ S2CID 17033286.
- S2CID 81983826.
- S2CID 220875296.
- ^ PMID 19236579.
- ^ S2CID 15579801.
- S2CID 17870808.
- .
- ISSN 0038-0717.
- S2CID 18149924.
- S2CID 15963708.
- hdl:1807/79634.
- hdl:10795/2323.
- ^ "University of Rhode Island GreenShare Factsheets: Endopyhte-Enhanced Grasses". Archived from the original on 2006-03-12. Retrieved June 14, 2009.
- PMID 18957585.
- PMID 20705658.
- PMID 25672844.
- OCLC 858878994.
- PMID 21764951.
- PMID 25865953.
- S2CID 13176570.
- ^ PMID 28959613.
- S2CID 219590658.
- ^ PMID 30205507.
- ^ S2CID 370022.
- PMID 26811095.
- PMID 21497643.
- ^ S2CID 29471199.
- PMID 23905700.
- PMID 23434859.
- ISBN 978-81-322-1574-5.
- ^ Meyer W, Torres M, White J (2012). Stier J, Horgan B, Bonos S (eds.). Chapter 20: Biology and Applications of Fungal Endophytes in Turfgrasses. American Society of Agronomy. pp. Chapter 20.
{{cite book}}:|work=ignored (help) - PMID 22111580.
- PMID 29528408.
- PMID 21236130.
- ^ PMID 27559722.
