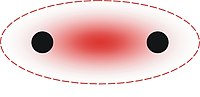
Ionic bonding
Ionic bonding is a type of
Clean ionic bonding — in which one atom or molecule completely transfers an electron to another — cannot exist: all ionic compounds have some degree of
Ionic compounds conduct electricity when molten or in solution, typically not when solid. Ionic compounds generally have a high melting point, depending on the charge of the ions they consist of. The higher the charges the stronger the cohesive forces and the higher the melting point. They also tend to be soluble in water; the stronger the cohesive forces, the lower the solubility.[3]
Overview
Atoms that have an almost full or almost empty
Properties of ionic bonds
- They are considered to be among the strongest of all types of chemical bonds. This often causes ionic compounds to be very stable.
- Ionic bonds have high bond energy. Bond energy is the mean amount of energy required to break the bond in the gaseous state.
- Most ionic compounds exist in the form of a crystal structure, in which the ions occupy the corners of the crystal. Such a structure is called a crystal lattice.
- Ionic compounds lose their crystal lattice structure and break up into ions when dissolved in polar solvent. This process is called solvation. The presence of these free ions makes aqueous ionic compound solutions good conductors of electricity. The same occurs when the compounds are heated above their melting point in a process known as melting.
Formation
Ionic bonding can result from a

For example, common
- Na + Cl → Na+ + Cl− → NaCl
However, to maintain charge neutrality, strict ratios between anions and cations are observed so that ionic compounds, in general, obey the rules of stoichiometry despite not being molecular compounds. For compounds that are transitional to the alloys and possess mixed ionic and metallic bonding, this may not be the case anymore. Many sulfides, e.g., do form non-stoichiometric compounds.
Many ionic compounds are referred to as salts as they can also be formed by the neutralization reaction of an Arrhenius base like NaOH with an Arrhenius acid like HCl
- NaOH + HCl → NaCl + H2O
The salt NaCl is then said to consist of the acid rest Cl− and the base rest Na+.
The removal of electrons to form the cation is endothermic, raising the system's overall energy. There may also be energy changes associated with breaking of existing bonds or the addition of more than one electron to form anions. However, the action of the anion's accepting the cation's valence electrons and the subsequent attraction of the ions to each other releases (lattice) energy and, thus, lowers the overall energy of the system.
Ionic bonding will occur only if the overall energy change for the reaction is favorable. In general, the reaction is exothermic, but, e.g., the formation of mercuric oxide (HgO) is endothermic. The charge of the resulting ions is a major factor in the strength of ionic bonding, e.g. a salt C+A− is held together by electrostatic forces roughly four times weaker than C2+A2− according to Coulomb's law, where C and A represent a generic cation and anion respectively. The sizes of the ions and the particular packing of the lattice are ignored in this rather simplistic argument.
Structures
Ionic compounds in the solid state form lattice structures. The two principal factors in determining the form of the lattice are the relative charges of the ions and their relative sizes. Some structures are adopted by a number of compounds; for example, the structure of the rock salt
Strength of the bonding
For a solid crystalline ionic compound the
The attractive forces defining the strength of ionic bonding can be modeled by Coulomb's Law. Ionic bond strengths are typically (cited ranges vary) between 170 and 1500 kJ/mol.[8][9]
Polarization power effects
Comparison with covalent bonding
In ionic bonding, the atoms are bound by attraction of oppositely charged ions, whereas, in
Purely ionic bonding cannot exist, as the proximity of the entities involved in the bonding allows some degree of sharing
Ionic character in covalent bonds can be directly measured for atoms having quadrupolar nuclei (2H, 14N, 81,79Br, 35,37Cl or 127I). These nuclei are generally objects of NQR nuclear quadrupole resonance and NMR nuclear magnetic resonance studies. Interactions between the nuclear quadrupole moments Q and the electric field gradients (EFG) are characterized via the nuclear quadrupole coupling constants
- QCC = e2qzzQ/h
where the eqzz term corresponds to the principal component of the EFG tensor and e is the elementary charge. In turn, the electric field gradient opens the way to description of bonding modes in molecules when the QCC values are accurately determined by NMR or NQR methods.
In general, when ionic bonding occurs in the solid (or liquid) state, it is not possible to talk about a single "ionic bond" between two individual atoms, because the cohesive forces that keep the lattice together are of a more collective nature. This is quite different in the case of covalent bonding, where we can often speak of a distinct bond localized between two particular atoms. However, even if ionic bonding is combined with some covalency, the result is not necessarily discrete bonds of a localized character.[2] In such cases, the resulting bonding often requires description in terms of a band structure consisting of gigantic molecular orbitals spanning the entire crystal. Thus, the bonding in the solid often retains its collective rather than localized nature. When the difference in electronegativity is decreased, the bonding may then lead to a semiconductor, a semimetal or eventually a metallic conductor with metallic bonding.
See also
- Coulomb's law
- Salt bridge (protein and supramolecular)
- Ionic potential
- Linear combination of atomic orbitals
- Hybridization
- Chemical polarity
- Ioliomics
- Electron configuration
- Aufbau principle
- Quantum numbers
References
- ISBN 978-0-9678550-9-7.
- ^ a b Seifert, Vanessa (27 November 2023). "Do bond classifications help or hinder chemistry?". chemistryworld.com. Retrieved 22 January 2024.
- ISBN 9781118165850.
- ISBN 0-85404-665-8
- ISBN 9780471972532
- PMID 27136957.
- OCLC 54091550.
- OCLC 903959750.
- ^ L. Pauling The Nature of the Chemical Bond (3rd ed., Oxford University Press 1960) p.98-100.