Nano-FTIR

Nano-FTIR (nanoscale Fourier transform infrared spectroscopy) is a

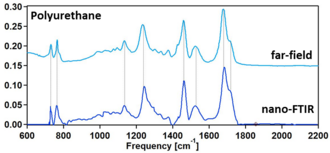
For organic compounds, polymers, biological and other soft matter, nano-FTIR spectra can be directly compared to the standard FTIR databases, which allows for a straightforward chemical identification and characterization.[4]
Nano-FTIR does not require special sample preparation and is typically performed under ambient conditions. It uses an AFM operated in
Basic principles

Nano-FTIR is based on s-SNOM, where the infrared beam from a light source is focused onto a sharp, typically metalized AFM tip and the backscattering is detected. The tip greatly enhances the illuminating IR light in the nanoscopic volume around its apex, creating a strong near field. A sample, brought into this near field, interacts with the tip electromagnetically and modifies the tip (back)scattering in the process. Thus by detecting tip scattering, one can obtain information about the sample.
Nano-FTIR detects the tip-scattered light interferometrically. The sample stage is placed into one arm of a conventional
Placement of the sample stage into one of the interferometer's arms (instead of outside of the interferometer as typically implemented in
History
Nano-FTIR was first described in 2005 in a patent by Ocelic and Hillenbrand as Fourier-transform spectroscopy of tip-scattered light with an asymmetric spectrometer (i.e. the tip/sample placed inside one of the interferometer arms).

The breakthrough in nano-FTIR came upon the development of high-power broadband mid-IR laser sources, which provided large spectral irradiance in a sufficiently large bandwidth (mW-level power in ~1000 cm-1 bandwidth)
Additional boost to the development of nano-FTIR came from the utilization of the synchrotron radiation that provide extreme bandwidth, yet at the expense of weaker IR spectral irradiance compared to broadband laser sources.[24][25][26][27]
Commercialization

The nano-FTIR technology has been commercialized by neaspec – a Germany-based spin-off company of the Max Planck Institute of Biochemistry founded by Ocelic, Hillenbrand and Keilmann in 2007 and based on the original patent by Ocelic and Hillenbrand.[11] The detection module optimized for broadband illumination sources was first made available in 2010 as a part of the standard neaSNOM microscope system. At this time, broadband IR-lasers have not been yet commercially available, however experimental broadband IR-lasers prove that the technology works perfect and that it has a huge application potential across many disciplines. The first nano-FTIR was commercially available in 2012 (supplied with still experimental broadband IR-laser sources), becoming the first commercial system for broadband infrared nano-spectroscopy. In 2015 neaspec develops and introduces Ultrafast nano-FTIR, the commercial version of ultrafast nano-spectroscopy. Ultrafast nano-FTIR is a ready-to-use upgrade for nano-FTIR to enable pump-probe nano-spectroscopy at best-in-class spatial resolution. The same year the development of a cryo-neaSNOM – the first system of its kind to enable nanoscale near-field imaging & spectroscopy at cryogenic temperatures – was announced.
Advanced capabilities
Synchrotron beamlines integration
Nano-FTIR systems can be easily integrated into synchrotron radiation beamlines. The use of synchrotron radiation allows for acquisition of an entire mid-infrared spectrum at once. Synchrotrons radiation has already been utilized in synchrotron infrared microscopectroscopy - the technique most widely used in biosciences, providing information on chemistry on microscales of virtually all biological specimens, like bone, plants, and other biological tissues.[28] Nano-FTIR brings the spatial resolution to 10-20 nm scale (vs. ~2-5 μm in microspectroscopy), which has been utilized for broadband spatially-resolved spectroscopy of crystalline[24][25] and phase-change[29] materials, semiconductors,[27] minerals,[30] biominerals and proteins.[26]
Ultrafast spectroscopy
Nano-FTIR is highly suitable for performing local ultrafast pump-probe spectroscopy due to intereferometric detection and an intrinsic ability to vary the probe delay time. It has been applied for studies of ultrafast nanoscale plasmonic phenomena in Graphene,[31][32] for performing nanospectroscopy of InAs nanowires with subcycle resolution[33] and for probing the coherent vibrational dynamics of nanoscopic ensembles.[6]
Quantitative studies
The availability of both amplitude and phase of the scattered field and theoretically well understood signal formation in nano-FTIR allow for the recovery of both real and imaginary parts of the dielectric function, i.e. finding the index of refraction and the extinction coefficient of the sample.[34] While such recovery for arbitrarily-shaped samples or samples exhibiting collective excitations, such as phonons, requires a resource-demanding numerical optimization, for soft matter samples (polymers, biological matter and other organic materials) the recovery of the dielectric function could often be performed in real time using fast semi-analytical approaches. One of such approaches is based on the Taylor expansion of the scattered field with respect to a small parameter that isolates the dielectric properties of the sample and allows for a polynomial representation of measured near-field contrast. With an adequate tip-sample interaction model[35] and with known measurement parameters (e.g. tapping amplitude, demodulation order, reference material, etc.), the sample permittivity can be determined as a solution of a simple polynomial equation[36]
Subsurface analysis
Near-field methods, including nano-FTIR, are typically viewed as a technique for surface studies due to short probing ranges of about couple tip radii (~20-50 nm). However it has been demonstrated that within such probing ranges, s-SNOM is capable of detecting subsurface features to some extents,[37][38][39][40] which could be used for the investigations of samples capped by thin protective layers,[41] or buried polymers,[42][43] among others.
As a direct consequence of being quantitative technique (i.e. capable of highly reproducible detection of both near-field amplitude & phase and well understood near-field interaction models), nano-FTIR also provides means for the quantitative studies of the sample interior (within the probing range of the tip near field, of course). This is often achieved by a simple method of utilizing signals recorded at multiple demodulation orders naturally returned by nano-FTIR in the process of
Operation in liquid
Nano-FTIR uses scattered IR light to obtain information about the sample and has the potential to investigate electrochemical interfaces in-situ/operando and biological (or other) samples in their natural environment, such as water. The feasibility of such investigations has already been demonstrated by acquisition of nano-FTIR spectra through a capping Graphene layer on top of a supported material or through Graphene suspended on a perforated silicon nitride membrane (using the same s-SNOM platform that nano-FTIR utilizes).[48][49]
Cryogenic environment
Reveling the fundamentals of phase transitions in superconductors, correlated oxides, Bose-Einstein condensates of surface polaritons, etc. require spectroscopic studies at the characteristically nanometer length scales and in cryogenic environment. Nano-FTIR is compatible with cryogenic s-SNOM that has already been utilized for reveling a nanotextured coexistence of metal and correlated Mott insulator phases in Vanadium oxide near the metal-insulator transition.[50]
Special atmosphere environments
Nano-FTIR can be operated in different atmospheric environments by enclosing the system into an isolated chamber or a glove box. Such operation has already been used for the investigation of highly reactive Lithium-ion battery components.[45]
Applications
Nano-FTIR has a plenitude of applications,[51] including polymers and polymer composites,[4] organic films,[52] semiconductors,[16][26][27][46] biological research (cell membranes, proteins structure, studies of single viruses),[2][26][53] chemistry and catalysis,[54] photochemistry,[55] minerals and biominerals,[53][26][30] geochemistry,[56] corrosion[57] and materials sciences,[5][23] low-dimensional materials,[58][32] photonics,[59][26] energy storage,[45] cosmetics, pharmacology and environmental sciences.[60]
Materials and chemical sciences
Nano-FTIR has been used for the nanoscale spectroscopic chemical identification of polymers[4] and nanocomposites,[20] for in situ investigation of structure and crystallinity of organic thin films,[52] for strain characterization and relaxation in crystalline materials[23] and for high-resolution spatial mapping of catalytic reactions,[54] among others.
Biological and pharmaceutical sciences
Nano-FTIR has been used for investigation of protein secondary structure, bacterial membrane,[26] detection and studies of single viruses and protein complexes.[26] It has been applied to the detection of biominerals in bone tissue.[53][26] Nano-FTIR, when coupled with THz light, can also be applied to cancer and burn imaging with high optical contrast.
Semiconductor industry and research
Nano-FTIR has been used for nanoscale free carrier profiling and quantification of free carrier concentration in semiconductor devices,[16] for evaluation of ion beam damage in nanoconstriction devices,[46] and general spectroscopic characterization of semiconductor materials.[27]
Theory
High-harmonic demodulation for background suppression
The nano-FTIR interferometrically detects light scattered from the tip-sample system, . The power at the detector can be written as[61]
where is the reference field. The scattered field can be written as
and is dominated by parasitic background scattering, , from the tip shaft, cantilever sample roughness and everything else which falls into the
where is the complex-valued number that is obtained by combining the lock-in amplitude, , and phase, , signals, is the n-th Fourier coefficient of the near-field contribution and C. C. stands for the complex conjugate terms. is the zeroth-order Fourier coefficient of the background contribution and is often called the multiplicative background because it enters the detector signal as a product with . It cannot be removed by the high-harmonic demodulation alone. In nano-FTIR the multiplicative background is eliminated as described below.
Asymmetric FTIR spectrometer
To acquire a spectrum, the reference mirror is continuously translated while recording the demodulated detector signal as a function of the reference mirror position , yielding an interferogram . This way the phase of the reference field changes according to for each spectral component of the reference field and the detector signal can thus be written as[62]
where is the reference field at zero delay . To obtain the nano-FTIR spectrum, , the interferogram is Fourier-transformed with respect to . The second term in the above equation does not depend on the reference mirror position and after the Fourier transformation contributes only to the DC signal. Thus for only the near-field contribution multiplied by the reference field stays in the acquired spectrum:
This way, besides providing the interferometric gain, the asymmetric interferometer utilized in nano-FTIR also eliminates the multiplicative background, which otherwise could be a source of various artifacts and is often overlooked in other s-SNOM based spectroscopies.
Normalization
Following the standard FTIR practice, spectra in nano-FTIR are normalized to those obtained on a known, preferably spectrally-flat reference material. This eliminates the generally unknown reference field and any instrumental functions, yielding spectra of the near-field contrast:
Near-field contrast spectra are generally complex-valued, reflecting on the possible phase delay of the sample-scattered field with respect to the reference. Near-field contrast spectra depend nearly exclusively on the dielectric properties of sample material and can be used for its identification and characterization.
Nano-FTIR absorption spectroscopy
For the purpose of describing near-field contrasts for optically thin samples composed of polymers, organics, biological matter and other soft matter (so called weak oscillators), the near-field signal to a good approximation can be expressed as:[36]
,
where is the surface response function that depends on the complex-valued dielectric function of the sample and can be also viewed as the reflection coefficient for evanescent waves that constitute the near field of the tip. That is, the spectral dependence of is determined exclusively by the sample reflection coefficient. The latter is purely real and acquires an imaginary part only in narrow spectral regions around the sample absorption lines. This means that the spectrum of an imaginary part of the near-field contrast resembles the conventional FTIR absorbance spectrum, , of the sample material:[4]. It is therefore convenient to define the nano-FTIR absorption , which directly relates to the sample absorbance spectrum:
It can be used for direct sample identification and characterization according to the standard FTIR databases without the need of modeling the tip-sample interaction.
For phononic and plasmonic samples in the vicinity of the corresponding surface surface resonances, the relation might not hold. In such cases the simple relation between and can not be obtained, requiring modeling of the tip-sample interaction for spectroscopic identification of such samples.[40]
Analytical and numerical simulations
Significant efforts have been put towards simulating nano-FTIR electric field and complex scattering signal through numerical methods[63] (using commercial proprietary software such as CST Microwave Studio, Lumerical FDTD, and COMSOL Multiphysics) as well as through analytical models[64] (such as through finite dipole and point dipole approximations). Analytical simulations tend to be more simplified and inaccurate, while numerical methods are more rigorous but computationally expensive.
References
- ^ PMID 19997423.
- ^ PMID 24301518.
- ^ PMID 26291869.
- ^ PMID 22703339.
- ^ hdl:10810/64521.
- ^ PMID 23387347.
- PMID 24721995.
- ^ Huth F (2015). Nano-FTIR - Nanoscale Infrared Near-Field Spectroscopy (Ph.D.). Universidad del Pais Vasco.
- ISSN 0003-6951.
- PMID 22214211.
- ^ a b WO patent 2007039210, Nenad Ocelic & Rainer Hillenbrand, "Optical device for measuring modulated signal light", published 2007-04-12
- PMID 19529536.
- PMID 15259740.
- PMID 34131580.
- .
- ^ PMID 21499314.
- PMID 22565729.
- PMID 23362918.
- .
- ^ PMID 28198384.
- S2CID 25305889.
- S2CID 49192831.
- ^ PMID 25321708.
- ^ PMID 23481749.
- ^ ISSN 0003-6951.
- ^ PMID 24803431.
- ^ PMID 25089414.
- ISBN 9783527600908.
- PMID 29273808.
- ^ PMID 29416052.
- S2CID 19561017.
- ^ S2CID 4278267.
- S2CID 119285417.
- PMID 26138665.
- PMID 19547189.
- ^ PMID 26282309.
- PMID 19498922.
- PMID 29092272.
- PMID 22274381.
- ^ S2CID 37170378.
- ISSN 0003-6951.
- S2CID 146790787.
- PMID 32620874.
- ^ PMID 24897380.
- ^ PMID 25375874.
- ^ S2CID 206737748.
- S2CID 53292498.
- S2CID 30158736.
- S2CID 196812291.
- .
- PMID 26262987.
- ^ PMID 24916130.
- ^ PMID 22563528.
- ^ S2CID 4452069.
- PMID 27966702.
- PMID 25487365.
- .
- S2CID 4253950.
- PMID 26112474.
- ISSN 1684-9981.
- ISSN 0003-6951.
- ^ Huth F (2015). Nano-FTIR - Nanoscale Infrared Near-Field Spectroscopy (Ph.D.). Universidad del Pais Vasco.
- ISSN 0003-6951.
- PMID 19547189.






























![{\textstyle {\rm {Im}}[\sigma (\omega )]\propto A(\omega )}](https://wikimedia.org/api/rest_v1/media/math/render/svg/628582574596a34c054ada53404bc9d222e802b4)




