Pauson–Khand reaction

The Pauson–Khand (PK) reaction is a chemical reaction, described as a [2+2+1] cycloaddition. In it, an alkyne, an alkene and carbon monoxide combine into a α,β-cyclopentenone in the presence of a metal-carbonyl catalyst.[1][2]
Ihsan Ullah Khand (1935–1980) discovered the reaction around 1970, while working as a postdoctoral associate with Peter Ludwig Pauson (1925–2013) at the University of Strathclyde in Glasgow.[3][4][5] Pauson and Khand's initial findings were intermolecular in nature, but the reaction has poor selectivity. Most modern applications instead apply the reaction for intramolecular ends.[6]
The traditional catalyst is stoichiometric amounts of dicobalt octacarbonyl, stabilized by a carbon monoxide atmosphere.[7] Catalytic metal quantities, enhanced reactivity and yield, or stereoinduction are all possible with the right chiral auxiliaries, choice of transition metal (Ti, Mo, W, Fe, Co, Ni, Ru, Rh, Ir and Pd), and additives.[8][9][10][11]
Mechanism
While the mechanism has not yet been fully elucidated, Magnus' 1985 explanation[12] is widely accepted for both mono- and dinuclear catalysts, and was corroborated by computational studies published by Nakamura and Yamanaka in 2001.[13] The reaction starts with dicobalt hexacarbonyl acetylene complex. Binding of an alkene gives a metallacyclopentene complex. CO then migratorily inserts into an M-C bond. Reductive elimination delivers the cyclopentenone. Typically, the dissociation of carbon monoxide from the organometallic complex is rate limiting.[8]
Selectivity
The reaction works with both terminal and internal alkynes, although internal alkynes tend to give lower yields. The order of reactivity for the alkene is
(strained cyclic) > (terminal) > (disubstituted) > (trisubstituted).
Tetrasubstituted alkenes and alkenes with strongly
With unsymmetrical alkenes or alkynes, the reaction is rarely

For mono-substituted alkenes, alkyne substituents typically direct: larger groups prefer the C2 position, and electron-withdrawing groups prefer the C3 position.

But the alkene itself struggles to discriminate between the C4 and C5 position, unless the C2 position is sterically congested or the alkene has a chelating heteroatom.
The reaction's poor selectivity is ameliorated in intramolecular reactions. For this reason, the intramolecular Pauson-Khand is common in total synthesis, particularly the formation of 5,5- and 6,5-membered fused bicycles.
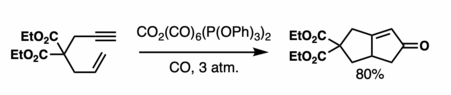
Generally, the reaction is highly syn-selective about the bridgehead hydrogen and substituents on the cyclopentane.

Appropriate chiral ligands or auxiliaries can make the reaction enantioselective (see § Amine N-oxides). BINAP is commonly employed.
Additives

Typical Pauson-Khand conditions are elevated temperatures and pressures in aromatic hydrocarbon (benzene, toluene) or ethereal (tetrahydrofuran, 1,2-dichloroethane) solvents. These harsh conditions may be attenuated with the addition of various additives.

Absorbent surfaces
Adsorbing the metallic complex onto silica or alumina can enhance the rate of decarbonylative ligand exchange as exhibited in the image below.[15][16] This is because the donor posits itself on a solid surface (i.e. silica).[clarification needed] Additionally using a solid support restricts conformational movement (rotamer effect).[17][18][19]
Lewis bases
Traditional catalytic aids such as phosphine ligands make the cobalt complex too stable, but bulky phosphite ligands are operable.
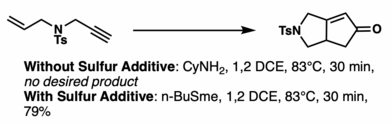
Lewis basic additives, such as n-BuSMe, are also believed to accelerate the decarbonylative ligand exchange process. However, an alternative view holds that the additives make olefin insertion irreversible instead.[20] Sulfur compounds are typically hard to handle and smelly, but n-dodecyl methyl sulfide[21] and tetramethylthiourea[22] do not suffer from those problems and can improve reaction performance.
Amine N-oxides
The two most common amine N-oxides are N-methylmorpholine N-oxide (NMO) and trimethylamine N-oxide (TMANO). It is believed that these additives remove carbon monoxide ligands via nucleophilic attack of the N-oxide onto the CO carbonyl, oxidizing the CO into CO2, and generating an unsaturated organometallic complex.[23][24] This renders the first step of the mechanism irreversible, and allows for more mild conditions. Hydrates of the aforementioned amine N-oxides have similar effect.[25][26][27]

N-oxide additives can also improve enantio- and diastereoselectivity, although the mechanism thereby is not clear.[28][29][30]

Alternative catalysts
The original Pauson-Khand catalyst is a low-oxidation-state cobalt complex unstable in air. Multinuclear cobalt catalysts like (Co)4(CO)12 and Co3(CO)9(μ3-CH) suffer from the same flaw,[31][32] although Takayama et al detail a reaction catalyzed by dicobalt octacarbonyl.[33]
One stabilization method is to generate the catalyst in situ. Chung reports that
Other metals

Substrate tolerance
In general allenes, support the Pauson–Khand reaction; regioselectivity is determined by the choice of metal catalyst. Density functional investigations show the variation arises from different transition state metal geometries.[41]
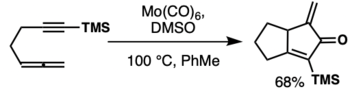
Heteroatoms are also acceptable: Mukai et al's total synthesis of physostigmine applied the Pauson–Khand reaction to a carbodiimide.[42]


An example of a newer version is the use of the chlorodicarbonylrhodium(I) dimer, [(CO)2RhCl]2, in the synthesis of (+)-phorbol by Phil Baran. In addition to using a rhodium catalyst, this synthesis features an intramolecular cyclization that results in the normal 5-membered α,β-cyclopentenone as well as 7-membered ring.[43]
Carbon monoxide generation in situ
Recently, several groups have published work avoiding the use of toxic carbon monoxide, and instead generate the cyclopentenone carbonyl motif from aldehydes, carboxylic acids, and formates. These examples typically employ rhodium as the organometallic transition metal, as it is commonly used in decarbonylation reactions. The decarbonylation and PK reaction occur in the same reaction vessel.[44][45]
See also
Further reading
For Khand and Pauson's perspective on the reaction:
- Khand, Ihsan U.; Knox, Graham R.; ISSN 0300-922X.
- Khand, Ihsan U.; Knox, Graham R.; ISSN 0300-922X.
- S2CID 84203764.
For a modern perspective:
- Hartwig, John F. (2010). Organotransition Metal Chemistry: from bonding to catalysis. Mill Valley, Calif.: University Science Books. OCLC 310401036– via Knovel.
- Ríos Torres, Ramón (2012). Rios Torres, Ramon (ed.). The Pauson-Khand reaction : scope, variations, and applications. Hoboken, N.J.: John Wiley & Sons. OCLC 774982574.
- Gibson, Susan E.; Stevenazzi, Andrea (2003). "The Pauson–Khand Reaction: The Catalytic Age Is Here!". PMID 12722067.
- Buchwald, Stephen L.; Hicks, Frederick A. (1999). "Pauson–Khand-type reactions". In Jacobsen, Eric N.; Pfaltz, Andreas; Yamamoto Hisashi (eds.). Comprehensive Asymmetric Catalysis. Vol. II. Berlin: Springer. pp. 491–513.
References
- ^ Pauson & Khand 1977.
- PMID 14737507.
- .
- ^ Khand et al. 1973a.
- ^ Khand et al. 1973b.
- ISSN 0022-3263.
- ^ Buchwald & Hicks 1999.
- ^ a b Hartwig 2010.
- ^ a b Ríos Torres 2012.
- ISBN 0471264180.
- ^ Gibson & Stevenazzi 2003.
- ISSN 0040-4020.
- PMID 11456770.
- OCLC 60792519.
- ISSN 0040-4039.
- ISSN 0300-9580.
- ISSN 0300-922X.
- S2CID 250841849.
- ISSN 0040-4039.
- PMID 16078280.
- S2CID 229387356.
- PMID 10814386.
- ISSN 0040-4039.
- doi:10.1139/v70-251.
- ISSN 0040-4020.
- ISSN 0022-3263.
- ISSN 0022-3263.
- ^ ISSN 0002-7863.
- ISSN 0040-4039.
- S2CID 196781210.
- S2CID 196736582.
- ISSN 0002-7863.
- ^ S2CID 10947595.
- ISBN 9781118942819.
- ISSN 0040-4039.
- ^ Nakcheol Jeong, Byung Ki Sung, Jin Sung Kim, Soon Bong Park,Sung Deok Seo, Jin Young Shin, Kyu Yeol In, Yoon Kyung Choi Pauson–Khand-type reaction mediated by Rh(I) catalysts Pure Appl. Chem., Vol. 74, No. 1, pp. 85–91, 2002. (Online article)
- .
- ^ Titanium:
- Hicks, Frederick A.; Buchwald, Stephen L. (1996-01-01). "Highly Enantioselective Catalytic Pauson−Khand Type Formation of Bicyclic Cyclopentenones". Journal of the American Chemical Society. 118 (46): 11688–11689. ISSN 0002-7863.
- Hicks, Frederick A.; Kablaoui, Natasha M.; Buchwald, Stephen L. (January 1996). "Titanocene-Catalyzed Cyclocarbonylation of Enynes to Cyclopentenones". Journal of the American Chemical Society. 118 (39): 9450–9451. ISSN 0002-7863.
- Zhang, Minghui; Buchwald, Stephen L. (January 1996). "A Nickel(0)-Catalyzed Process for the Transformation of Enynes to Bicyclic Cyclopentenones". The Journal of Organic Chemistry. 61 (14): 4498–4499. PMID 11667365.
- Hicks, Frederick A.; Buchwald, Stephen L. (1996-01-01). "Highly Enantioselective Catalytic Pauson−Khand Type Formation of Bicyclic Cyclopentenones". Journal of the American Chemical Society. 118 (46): 11688–11689.
- ^
- Negishi, Eiichi; Holmes, Steven J.; Tour, James M.; Miller, Joseph A. (1985-04-01). "Metal promoted cyclization. 7. Zirconium-promoted bicyclization of enynes". Journal of the American Chemical Society. 107 (8): 2568–2569. ISSN 0002-7863.
- Negishi, Eiichi; Holmes, Steven J.; Tour, James M.; Miller, Joseph A.; Cederbaum, Fredrik E.; Swanson, Douglas R.; Takahashi, Tamotsu (April 1989). "Metal-promoted cyclization. 19. Novel bicyclization of enynes and diynes promoted by zirconocene derivatives and conversion of zirconabicycles into bicyclic enones via carbonylation". Journal of the American Chemical Society. 111 (9): 3336–3346. ISSN 0002-7863.
- Negishi, Eiichi; Holmes, Steven J.; Tour, James M.; Miller, Joseph A. (1985-04-01). "Metal promoted cyclization. 7. Zirconium-promoted bicyclization of enynes". Journal of the American Chemical Society. 107 (8): 2568–2569.
- ISSN 0002-7863.
- PMID 26005240.
- PMID 16381573.
- PMID 27007853.
- .
- .
