Migratory insertion
In
Overview
In the migratory insertion, a ligand that is viewed as an
The anionic ligand can be: H− (
Diverse reactions apply to the migratory insertion. One mechanism involves the attack of the anionic ligand on the electrophilic part of the neutral ligand (the anionic ligand migrates to the neutral ligand). The other mechanism involves the neutral ligand inserts itself between the metal and the anionic ligand.
CO insertion
The insertion of
Mechanism
CO inserts into a metal-
CO insertion does not always involve migration. Treatment of CpFe(L)(CO)CH3 with 13CO yields a mix of both alkyl migration products and products formed by true insertion of bound
Alkyl derivatives of

Effects on reaction rates
- Steric effects strain – Increasing the steric strain of the chelate backbone in square planar complexes pushes the carbonyl and methyl groups closer together, increasing the reactivity of insertion reactions.[5]
- Oxidation state – Oxidation of the metal tends to increase insertion reaction rates. The main rate-limiting step in the mechanism is the migration of the methyl group onto a carbonyl ligand, oxidizing the metal by imparting a greater partial positive charge on the acetyl carbon, and thus increasing the rate of reaction.[6]
- Lewis acids – Lewis acids also increase the reaction rates, for reasons similar to metal oxidation increasing the positive charge on the carbon. Lewis acids bind to the CO oxygen and remove charge, increasing the electrophilicity of the carbon. This can increase the reaction rate by a factor of up to 108, and the complex formed is stable enough that the reaction proceeds even without additional CO to bind to the metal.[6]
- Electronegativity of the leaving group - Increasing the electronegativity of the leaving alkyl group stabilizes the metal-carbon bond interaction and thus increases the activation energy required for migration, decreasing the reaction rate.[7]
- bidentate phosphorus- or nitrogen-bound ligand. This reaction proceeds in much greater yield when the methyl group is trans-P and the CO trans-N, owing to the higher trans-influence of the more electronegative nitrogen.[5]
Reverse reaction
- RCHO → RH + CO
The reaction is not widely practiced in part because the
- RhCl(PPh3)3 + RCHO → RhCl(CO)(PPh3)2 + RH + PPh3
Please see
Insertion of other oxides
Many electrophilic oxides insert into metal carbon bonds; these include sulfur dioxide, carbon dioxide, and nitric oxide. These reactions have limited or no practical significance, but are of historic interest. With transition metal alkyls, these oxides behave as electrophiles and insert into the bond between metals and their relatively nucleophilic alkyl ligands. As discussed in the article on Metal sulfur dioxide complexes, the insertion of SO2 has been examined in particular detail. SO2 inserts to give both O-sulphinates and S-sulphinates, depending on the metal centre.[10] With square planar alkyl complexes, a pre-equilibrium is assumed involving formation of an adduct.[11]
Insertion of alkenes into metal-carbon bonds
The insertion of

Steps in alkene polymerization. Step i involves binding of the monomer to the metal and step ii involves the migratory insertion step. These steps, which alternate from one side of the metal center to the other, are repeated many times for each polymer chain. The box represents a vacant (or extremely labile) coordination site.
Mechanism
Factors affecting the rate of olefin insertions include the formation of the cyclic, planar, four-center transition state involving incipient formation of a bond between the metal and an olefin carbon. From this transition state, it can be seen that a partial positive charge forms on the β-carbon with a partial negative charge formed on the carbon initially bonded to the metal. This polarization explains the subsequently observed formation of the bond between the negatively charged carbon/hydrogen and the positively charged β-carbon as well as the simultaneous formation of the metal-α-carbon bond. This transition state also highlights the two factors that most strongly contribute to the rate of olefin insertion reactions: (i) orbital overlap of the alkyl group initially attached to the metal and (ii) the strength of the metal-alkyl bond. With greater orbital overlap between the partially positive β-carbon and the partially negative hydrogen/alkyl group carbon, the formation of the new C-C bond is facilitated. With increasing strength of the metal-alkyl bond, the breaking of the bond between the metal and the hydrogen/alkyl carbon bond to form the two new bonds with the α-carbon and β-carbon (respectively) is slower, thus decreasing the rate of the insertion reaction.[12]
Insertion of alkenes into M–H bonds
The insertion of alkenes into metal-hydrogen bonds is a key step in hydrogenation and hydroformylation reactions. The reaction involves the alkene and the hydride ligands combining within the coordination sphere of a catalyst. In hydrogenation, the resulting alkyl ligand combines with a second hydride to give the alkane. Analogous reactions apply to the hydrogenation of alkynes: an alkenyl ligand combines with a hydride to eliminate an alkene.

Mechanism
In terms of mechanism, the insertion of alkenes into M–H bond and into M–C bonds are described similarly. Both involve four-membered transition states that place the less substituted carbon on the metal.
The reverse of olefin insertion into a metal-hydrogen bond is
Industrial applications
Carbonylation
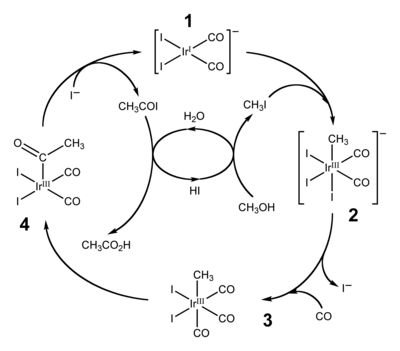
Two widely employed applications of migratory insertion of carbonyl groups are hydroformylation and the production of acetic acid by carbonylation of methanol. The former converts alkenes, hydrogen, and carbon monoxide into aldehydes. The production of acetic acid by carbonylation proceeds via two similar industrial processes. More traditional is the Monsanto acetic acid process, which relies on a rhodium-iodine catalyst to transform methanol into acetic acid. This process has been superseded by the Cativa process which uses a related iridium catalyst, [Ir(CO)2I2]− (1).[14][15] By 2002, worldwide annual production of acetic acid stood at 6 million tons, of which approximately 60% is produced by the Cativa process.[14]
The Cativa process
Alkene polymerization
Industrial applications of alkene insertions include metal-catalyzed routes to polyethylene and polypropylene. Typically these conversions are catalyzed heterogeneously by titanium trichloride which are activated by aluminium alkyls. This technology is known as Ziegler–Natta catalysts.[16] In these reactions, ethylene coordinates to titanium metal followed by its insertion. These steps can be repeated multiple times, potentially leading to high molecular weight polymers.
References
- ISBN 978-3-527-29390-2.
- ISBN 978-1-891389-53-5.
- ISBN 978-81-261-1898-4.
- ^ .
- ^ .
- ^ .
- .
- S2CID 207119793.
- .
- ISBN 978-0-471-62978-8.
- .
- .
- ISBN 978-0-470-25762-3.
- ^ Platinum Metals Review. 44 (3): 94–105.
- .
- ISBN 978-0-444-53215-2.