Midodrine
 | |
| Clinical data | |
|---|---|
| Trade names | Amatine, Proamatine, Gutron, others |
| Other names | 2-amino-N-[2-(2,5-dimethoxyphenyl)-2-hydroxy-ethyl]-acetamide |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a602023 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
JSmol) | |
| Chirality | Racemic mixture |
| |
| |
| | |
Midodrine is a
Medical uses
Midodrine is indicated for the treatment of symptomatic orthostatic hypotension. It can reduce dizziness and faints by about a third, but can be limited by troublesome
Midodrine has been used in the complications of cirrhosis. It is also used with octreotide for hepatorenal syndrome; the proposed mechanism is constriction of splanchnic vessels and dilation of renal vasculature. Studies have not been sufficiently well conducted to show a clear place for midodrine.[9]
Contraindications
Midodrine is contraindicated in patients with severe organic heart disease, acute kidney disease,
Side effects
Headache, feeling of pressure/fullness in the head, vasodilation/flushing face, scalp tingling, confusion/thinking abnormality, dry mouth, nervousness/anxiety and rash.[11]
Pharmacology
Mechanism of action
Midodrine is a

Pharmacokinetics
After oral administration, midodrine is rapidly absorbed. The plasma levels of the prodrug peak after about half an hour, and decline with a half-life of approximately 25 minutes, while the metabolite reaches peak blood concentrations about 1 to 2 hours after a dose of midodrine and has a half-life of about 3 to 4 hours. The absolute bioavailability of midodrine (measured as desglymidodrine) is 93%.[12]
Chemistry
Midodrine is an odorless, white, crystalline powder, soluble in water and sparingly soluble in methanol.[13]
Stereochemistry
Midodrine contains a stereocenter and consists of two
| Enantiomers of midodrine | |
|---|---|
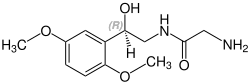 (R)-midodrine CAS number: 133163-25-4 |
 (S)-midodrine CAS number: 133267-39-7 |
Synthesis
Acylation of

References
- ^ "Proamatine- midodrine hydrochloride tablet". DailyMed. Retrieved 14 August 2021.
- ^ U.S. proposes withdrawal of Shire hypotension drug, 16 August 2010.
- ^ O'Riordan M. "FDA recommends withdrawal of midodrine". Food and Drug Administration. FDA proposes withdrawal of low blood pressure drug [press release]. August 16, 2010. TheHeart.org. Retrieved 1 April 2011.
- ^ Midodrine (ProAmatine, generic) Proposed Market Withdrawal – Update 10 September 2010.
- ^ Shire plc. "Shire Provides Update on ProAmatine® (midodrine HCl)". www.prnewswire.com.
- S2CID 5439767.
- S2CID 49674644.
- PMID 15280522.
- S2CID 207263346.
- ^ "Midodrine - FDA prescribing information, side effects and uses". Drugs.com. Retrieved 30 June 2022.
- ^ "Midodrine (Oral Route) Side Effects - Mayo Clinic". www.mayoclinic.org.
- ^ "Midodrine".
- ^ "DailyMed - MIDODRINE HCL- midodrine hydrochloride tablet". DailyMed. Retrieved 5 January 2023.
- ISBN 978-3-946057-10-9, S. 196.
- ISSN 1381-1169.
- ISBN 978-0-12-818656-5.
- ^ DE 2506110, Zoelss G, "Phenylethanolamine derivs prepn. - by reducing azides, useful as hypertensives", issued 21 April 1983, assigned to Lentia GmbH.
- ^ K. Wismayr et al., AT 241435; eidem, U.S. patent 3,340,298 (1965, 1967 both to Chemie Linz Ag).
- ^ Zoelss & W. Karl-Anton Ing DE 2523735 (1974 to Lentia GmbH).
External links
![]() Media related to Midodrine at Wikimedia Commons
Media related to Midodrine at Wikimedia Commons
