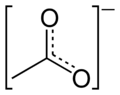
Acetate

| |
| Names | |
|---|---|
| IUPAC name
Acetate
| |
| Systematic IUPAC name
Ethanoate | |
| Identifiers | |
3D model (
JSmol ) |
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C 2H 3O− 2 | |
Conjugate acid
|
Acetic acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
An acetate is a
3CO−
2, or CH
3COO−
.
Most of the approximately 5 million tonnes of acetic acid produced annually in industry are used in the production of acetates, which usually take the form of
Nomenclature and common formula
When part of a
3CO−
2).[citation needed] It is not to be confused with the symbol of actinium, the first element of the actinide series; context guides disambiguation. For example, the formula for sodium acetate might be abbreviated as "NaOAc", rather than "NaC2H3O2". Care should also be taken to avoid confusion with peracetic acid
Although its systematic name is ethanoate (/ɪˈθænoʊ.eɪt/), the common acetate remains the preferred IUPAC name.[4]
Salts

The acetate
- CH3COOH ⇌ CH3COO− + H+
Many acetate salts are ionic, indicated by their tendency to dissolve well in water. A commonly encountered acetate in the home is sodium acetate, a white solid that can be prepared by combining vinegar and sodium bicarbonate ("bicarbonate of soda"):
- CH3COOH + NaHCO3 → CH3COO−Na+ + H2O + CO2
Transition metals can be complexed by acetate. Examples of acetate complexes include chromium(II) acetate and basic zinc acetate.
Commercially important acetate salts are aluminium acetate, used in dyeing, ammonium acetate, a precursor to acetamide, and potassium acetate, used as a diuretic. All three salts are colourless and highly soluble in water.[5]
Esters
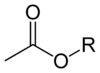
Acetate esters have the general formula CH3CO2R, where R is an organyl group. The esters are the dominant forms of acetate in the marketplace. Unlike the acetate salts, acetate esters are often liquids, lipophilic, and sometimes volatile. They are popular because they have inoffensive, often sweet odors, they are inexpensive, and they are usually of low toxicity.
Almost half of acetic acid production is consumed in the production of vinyl acetate, precursor to polyvinyl alcohol, which is a component of many paints. The second largest use of acetic acid is consumed in the production of cellulose acetate. In fact, "acetate" is jargon for cellulose acetate, which is used in the production of fibres or diverse products, e.g. the acetate discs used in audio record production. Cellulose acetate can be found in many household products. Many industrial solvents are acetates, including methyl acetate, ethyl acetate, isopropyl acetate, ethylhexyl acetate. Butyl acetate is a fragrance used in food products.[5]
Acetate in biology
Acetate is a common anion in biology. It is mainly utilized by organisms in the form of acetyl coenzyme A.[6]
Acetate has known
Fermentation acetyl CoA to acetate
Pyruvate is converted into acetyl-coenzyme A (acetyl-CoA) by the enzyme pyruvate dehydrogenase. This acetyl-CoA is then converted into acetate in E. coli, whilst producing ATP by substrate-level phosphorylation. Acetate formation requires two enzymes: phosphate acetyltransferase and acetate kinase.[10]

acetyl-CoA + phosphate → acetyl-phosphate + CoA
acetyl-phosphate + ADP → acetate + ATP
Fermentation of acetate
Acetic acid can also undergo a
- CH3COO− + H+ → CH4 + CO2 ΔG° = −36 kJ/mol
This
Structures
-
Space-filling model of the acetate anion
-
resonance hybrid of the acetate anion
-
canonical forms of the acetate anion
See also
- Acetylation
- Cellulose acetate
- Copper(II) acetate
- Fermentation (biochemistry)
- Sodium acetate
- Mixed acid fermentation
- Acetic acid
- Acetyl chloride
- Zinc acetate
References
- ^ ISBN 0-669-04529-2.
- ISBN 0-85404-438-8. p. 63. Electronic version.
- S2CID 55102299.
- ^ R-9.1 Trivial and semisystematic names retained for naming organic compounds Archived 2014-02-08 at the Wayback Machine, A Guide to IUPAC Nomenclature of Organic Compounds, IUPAC Commission on Nomenclature of Organic Chemistry
- ^
- ISBN 1-57259-153-6.
- PMID 21209842.
- ^ 'Is coffee the real cure for a hangover?' by Bob Holmes, New Scientist, Jan. 15 2011, p. 17.
- PMID 35085362.
- PMID 21097882.
- PMID 1512186.
- ^ Vogels, G. D.; Keltjens, J. T.; Van Der Drift, C. (1988). "Biochemistry of methane production". In Zehnder A.J.B. (ed.). Biology of anaerobic microorganisms. New York: Wiley. pp. 707–770.