Ammonium formate

| |

| |

| |
| Names | |
|---|---|
| IUPAC name
Ammonium formate
| |
| Identifiers | |
3D model (
JSmol ) |
|
| ChemSpider | |
ECHA InfoCard
|
100.007.959 |
PubChem CID
|
|
RTECS number
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| CH5NO2 | |
| Molar mass | 63.056 g·mol−1 |
| Appearance | White monoclinic crystals, deliquescent
|
| Odor | Slightly ammoniac |
| Density | 1.26 g/cm3[1] |
| Melting point | 116 °C (241 °F; 389 K) |
| Boiling point | 180 °C (356 °F; 453 K) decomposes[2] |
| (grams per 100g of water)102g(0 °C) 142.7 g (20 °C) 202.4 g (40 °C) 516 g (80 °C)[2] | |
| Solubility in other solvents | Soluble in liquid ammonia, alcohol, diethyl ether[2] |
| Thermochemistry | |
Std enthalpy of (ΔfH⦵298)formation |
−556.18 kJ/mol |
| Hazards | |
| GHS labelling: | |
 [1] [1]
| |
| Warning | |
| H315, H319, H335[1] | |
| P261, P305+P351+P338[1] | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
410 mg/kg (mice, intravenous)[2] |
| Safety data sheet (SDS) | JT Baker MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
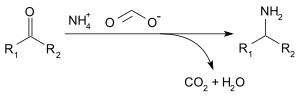
Ammonium formate, NH4HCO2, is the
Reductive amination
Acetone can be transformed into isopropylamine as follows:[citation needed]
- CH3C(O)CH3 + 2 HCO2− +NH4 → (CH3)2CHNHCHO + 2 H2O + NH3 + CO2
- (CH3)2CHNHCHO + H2O → (CH3)2CHNH2 + HCO2H
Uses
Pure ammonium formate decomposes into
Ammonium formate can also be used in palladium on carbon (Pd/C) reduction of functional groups. In the presence of Pd/C, ammonium formate decomposes to hydrogen, carbon dioxide, and ammonia. This hydrogen gas is adsorbed onto the surface of the palladium metal, where it can react with various functional groups. For example, alkenes can be reduced to alkanes, formaldehyde to methanol, and nitro compounds to amines.[3][4] Activated single bonds to heteroatoms can also be replaced by hydrogens (hydrogenolysis).
Ammonium formate can be used for
Ammonium formate can be used as a mobile phase additive in
Reactions
When heated, ammonium formate eliminates water, forming formamide. Upon further heating, it forms hydrogen cyanide (HCN) and water. A side reaction of this is the decomposition of formamide to carbon monoxide (CO) and ammonia.
References
- ^ a b c d Sigma-Aldrich Co., Ammonium formate. Retrieved on 2014-06-10.
- ^ a b c d "Ammonium formate".
- hdl:2027.42/25034.
- ISBN 978-0-471-72091-1.
- PMID 18909189.
External links
![]() Media related to Ammonium formate at Wikimedia Commons
Media related to Ammonium formate at Wikimedia Commons