Apolipoprotein AI
Ensembl | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| UniProt | |||||||||
| RefSeq (mRNA) | |||||||||
| RefSeq (protein) |
| ||||||||
| Location (UCSC) | Chr 11: 116.84 – 116.84 Mb | Chr 9: 46.14 – 46.14 Mb | |||||||
| PubMed search | [3] | [4] | |||||||
| View/Edit Human | View/Edit Mouse |
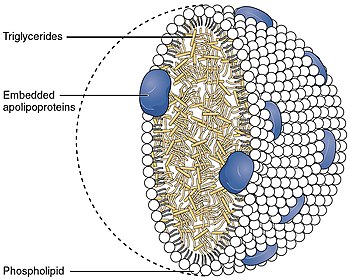
Apolipoprotein AI (Apo-AI) is a protein that in humans is encoded by the APOA1 gene.[5][6] As the major component of HDL particles, it has a specific role in lipid metabolism.
Structure
APOA1 is located on chromosome 11, with its specific location being 11q23-q24. The gene contains 4 exons.[7] The encoded apolipoprotein AI, is a 28.1 kDa protein composed of 243 amino acids; 21 peptides have been observed through mass spectrometry data.[8][9] Due to alternative splicing, there exists multiple transcript variants of APOA1, including at least one which encodes a Apo-AI preprotein.[7]
Function
Apolipoprotein AI is the major protein component of
Chylomicrons secreted from the intestinal enterocyte also contain Apo-AI, but it is quickly transferred to HDL in the bloodstream.[11]
The protein, as a component of HDL particles, enables efflux of fat molecules by accepting fats from within cells (including macrophages within the walls of arteries which have become overloaded with ingested fats from oxidized LDL particles) for transport (in the water outside cells) elsewhere, including back to LDL particles or to the liver for excretion.
It is a cofactor for
Apo-AI is often used as a biomarker for prediction of cardiovascular diseases. The ratio apoB-100/apoA-I (i.e. LDL & larger particles vs. HDL particles), NMR measured lipoprotein (
Apo-AI is routinely measured using immunoassays such as
Applications
Apo-AI can be used to create in vitro lipoprotein nanodiscs for cell-free membrane expression systems.[14]
Clinical significance
Activity associated with high HDL-C and protection from heart disease
As a major component of the high-density lipoprotein complex (protective "fat removal" particles), Apo-AI helps to clear fats, including cholesterol, from white blood cells within artery walls, making the white blood cells (WBCs) less likely to become fat overloaded, transform into foam cells, die and contribute to progressive atheroma. Five of nine men found to carry a mutation (E164X) who were at least 35 years of age had developed premature coronary artery disease.[15] One of four mutants of Apo-AI is present in roughly 0.3% of the Japanese population, but is found in 6% of those with low HDL cholesterol levels.[16]
Recombinant Apo-AI Milano dimers formulated into liposomes can reduce atheromas in animal models by up to 30%.[20] Apo-AI Milano has also been shown in small clinical trials to have a statistically significant effect in reducing (reversing) plaque build-up on arterial walls.[21][22]
In human trials the reversal of plaque build-up was measured over the course of five weeks.[21][23]
Novel haplotypes within apolipoprotein AI-CIII-AIV gene cluster
A study from 2008 describes two novel susceptibility haplotypes, P2-S2-X1 and P1-S2-X1, discovered in ApoAI-CIII-AIV gene cluster on chromosome 11q23, which confer approximately threefold higher risk of coronary heart disease in normal[24] as well as in the patients having type 2 diabetes mellitus.[25]
Role in other diseases
A G/A
Apo-AI binds to
In one study, a decrease in Apo-AI levels was detected in schizophrenia patients' CSF, brain and peripheral tissues.[30]
Epistatic impact of Apo-AI
Apolipoprotein AI and ApoE interact epistatically to modulate triglyceride levels in coronary heart disease patients. Individually, neither Apo-AI nor ApoE was found to be associated with triglyceride (TG) levels, but pairwise epistasis (additive x additive model) explored their significant synergistic contributions with raised TG levels (P<0.01). [31]
Factors affecting Apo-AI activity
In a study from 2005 it was reported, that Apo-AI production is decreased by calcitriol. It was concluded, that this regulation happens on transcription level: calcitriol alters yet unknown coactivators or corepressors, resulting in repression of APOA1 promoter activity. Simultaneously, Apo-AI production was increased by vitamin D antagonist, ZK-191784.[32]
Exercise or statin treatment may cause an increase in HDL-C levels by inducing Apo-AI production, but this depends on the G/A promoter polymorphism.[33]
Interactions
Apolipoprotein A1 has been shown to
Potential binding partners
Apolipoprotein AI binding precursor, a relative of APOA-1 abbreviated APOA1BP, has a predicted biochemical interaction with carbohydrate kinase domain containing protein. The relationship between these two proteins is substantiated by cooccurance across genomes and coexpression.[37] The ortholog of CARKD in E. coli contains a domain not present in any eukaryotic ortholog. This domain has a high sequence identity to APOA1BP. CARKD is a protein of unknown function, and the biochemical basis for this interaction is unknown.
Interactive pathway map
Click on genes, proteins and metabolites below to link to respective articles. [§ 1]
- ^ The interactive pathway map can be edited at WikiPathways: "Statin_Pathway_WP430".
See also
- Apolipoprotein B
- Cardiovascular disease
- ApoA-1 Milano
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000118137 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000032083 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- PMID 6294659.
- S2CID 22613512.
- ^ a b c "Entrez Gene: APOA1 apolipoprotein A1".
- PMID 23965338.
- ^ "Apolipoprotein A-IV". Cardiac Organellar Protein Atlas Knowledgebase (COPaKB). Archived from the original on 5 March 2016. Retrieved 25 March 2015.
- S2CID 213180689.
- S2CID 1989187.
- PMID 3047170.
- S2CID 26567691.
- PMID 31333463.
- PMID 16023124.
- PMID 9931341.
- ^ "The Long Saga of Apo-A1 Milano | in the Pipeline". 16 November 2016.
- PMID 6785551.
- PMID 15805548.
- S2CID 75941726.
- ^ a b "Apo A-I-Milano Trial: Where are we now?". Cleveland Clinic. Retrieved 26 July 2008.
- PMID 14600188.
- ^ "Apo A-I Milano". Cedars-Sinai Heart Institute. Archived from the original on 21 December 2007. Retrieved 26 July 2008.
- PMID 17825930.
- S2CID 23793589.
- S2CID 42148248.
- S2CID 30014992.
- PMID 17075859.
- PMID 15188057.
- S2CID 5576909.
- PMID 18378026.
- PMID 16236546.
- PMID 12801612.
- PMID 12084722.
- PMID 11254757.
- PMID 9469594.
- ^ "STRING: Known and Predicted Protein-Protein Interactions". Archived from the original on 18 July 2011.
External links
- Apolipoprotein+A-I at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Applied Research on Apolipoprotein-A1
- Human APOA1 genome location and APOA1 gene details page in the UCSC Genome Browser.
- Overview of all the structural information available in the PDB for UniProt: P02647 (Human Apolipoprotein A-I) at the PDBe-KB.
- Overview of all the structural information available in the PDB for UniProt: Q00623 (Mouse Apolipoprotein A-I) at the PDBe-KB.