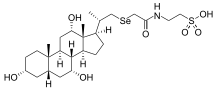
SeHCAT
| |
| Names | |
|---|---|
| IUPAC name
(75Se)-2-[[[[(3α,5α,7α,12α,20S)-3,7,12-trihydroxy-20-methylpregnan-21-yl]seleno]acetyl]amino]ethanesulfonic acid,
| |
| Other names
23-Seleno-25-homo-tauro-cholic acid; Selenium homocholic acid taurine; Tauroselcholic acid
| |
| Identifiers | |
3D model (
JSmol ) |
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C26H45NO7SSe | |
| Molar mass | 594.68 g·mol−1 |
| Pharmacology | |
| V09DX01 (WHO) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
SeHCAT (23-seleno-25-homotaurocholic acid, selenium homocholic acid taurine, or tauroselcholic acid) is a drug used in a clinical test to diagnose bile acid malabsorption.[1]
Development
SeHCAT is a taurine-conjugated
SeHCAT has been shown to be absorbed from the gut and excreted into the bile at the same rate as
Procedure
A capsule containing radiolabelled 75SeHCAT (with 370 kBq of Selenium-75 and less than 0.1 mg SeHCAT) is taken orally with water, to ensure passage of the capsule into the gastrointestinal tract. The physical half life of 75Se is approximately 118 days; activity is adjusted to a standard reference date.[citation needed]
Patients may be given instructions to fast prior to capsule administration; there is significant variation in clinical practice in this regard.[5] The effective dose of radiation for an adult given 370 kBq of SeHCAT is 0.26 mSv.[6] (For comparison, the radiation exposure from an abdominal CT scan is quoted at 5.3 mSv and annual background exposure in the UK 1-3 mSv.[7]) Measurements were originally performed with a whole-body counter but are usually performed now with an uncollimated gamma camera. The patient is scanned supine or prone with anterior and posterior acquisition from head to thigh 1 to 3 hours after taking the capsule. Scanning is repeated after 7 days. Background values are subtracted and care must be taken to avoid external sources of radiation in a nuclear medicine department.
From these measurements, the percent retention of SeHCAT at 7 days is calculated. A 7-day SeHCAT retention value greater than 15% is considered to be normal, with values less than 15% signifying excessive bile acid loss, as found in bile acid malabsorption.
With more frequent measurements, it is possible to calculate SeHCAT retention whole-body half-life; this is not routinely measured in a clinical setting. A half-life of greater than 2.8 days has been quoted as normal.[8]
Clinical use
The SeHCAT test is used to investigate patients with suspected
A similar picture of
There have been at least 18 studies of the use of SeHCAT testing in diarrhea-predominant irritable bowel syndrome patients. When these data were combined, 32% of 1223 patients had a SeHCAT 7-day retention of less than 10%, and 80% of these reported a response to cholestyramine, a bile acid sequestrant.[12]
