Lithium platinate
| |
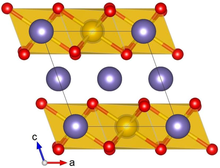
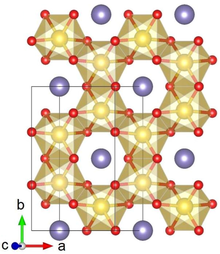 Crystal structure with Pt shown in yellow, Li in purple and O in red
| |
 Scale bar 1 mm[1]
| |
| Names | |
|---|---|
| Preferred IUPAC name
Lithium platinate | |
| Identifiers | |
3D model (
JSmol ) |
|
| |
| |
| Properties | |
| Li2PtO3 | |
| Appearance | Yellow crystals |
| Band gap | 2.3 eV[2] |
| Structure | |
| Monoclinic, C2/m[2] | |
a = 5.1836(2) Å, b = 8.9726(3) Å, c = 5.1113(1) Å α = 90°, β = 109.864(2)°, γ = 90°
| |
Formula units (Z)
|
4 |
| Related compounds | |
Other anions
|
Lithium iridate, lithium ruthenate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Lithium platinate, Li2PtO3, is a chemical compound of lithium, platinum and oxygen. It is a semiconductor with a layered honeycomb crystal structure and a band gap of 2.3 eV, and can be prepared by direct calcination of Pt metal and lithium carbonate at ca. 600 °C.[3] Lithium platinate is a potential lithium-ion battery electrode material,[2][4] though this application is hindered by the high costs of Pt, as compared to the cheaper Li2MnO3 alternative.[5]
References
- PMID 27748402.
- ^ .
- .
- .
- ISBN 978-0-387-34445-4.
