Manganese(II) iodide
| |

| |
| |
| Names | |
|---|---|
| IUPAC name
Manganese(II) iodide
| |
| Identifiers | |
3D model (
JSmol ) |
|
| ChemSpider | |
ECHA InfoCard
|
100.029.274 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| MnI2 | |
| Molar mass | 308.747 g/mol |
| Appearance | pink crystalline |
| Density | 5.01 g/cm3 |
| Melting point | 701 °C (1,294 °F; 974 K) (anhydrous) 80 °C (tetrahydrate) |
| Boiling point | 1,033 °C (1,891 °F; 1,306 K) |
| soluble | |
| +14,400·10−6 cm3/mol | |
| Structure | |
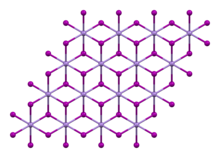
Rhombohedral, hP3 , SpaceGroup = P-3m1, No. 164
| |
| octahedral | |
| Hazards[1] | |
| GHS labelling: | |

| |
| Danger | |
| H360 | |
| P201, P202, P281, P308+P313, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | non-flammable |
| Related compounds | |
Other anions
|
Manganese(II) fluoride Manganese(II) chloride Manganese(II) bromide |
Other cations
|
Iron(II) iodide Cobalt(II) iodide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
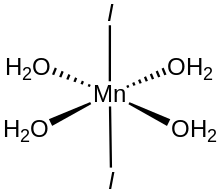
Manganese(II) iodide is the chemical compound composed of manganese and iodide with the formula MnI2(H2O)n. The tetrahydrate is a pink solid while the anhydrous derivative is beige.[2] Both forms feature octahedral Mn centers. Unlike MnCl2(H2O)4 and MnBr2(H2O)4 which are cis, MnI2(H2O)4 is trans.[3]
Preparation
Anhydrous MnI2 is prepared from the elements:[4]
- Mn + I2 → MnI2
The tetrahydrate can be prepared by treating
Properties
Samples turn brown in air under the influence of light as a result of the oxidation of the iodide ion to iodine.[5] It has a trigonal crystal structure of the cadmium iodide type (polytype 2H)[6][7] with the space group P3m1 (space group no. 164). It dissolves in water and decomposes.[5] The tetrahydrate has a monoclinic crystal structure with the space group P21/c (No. 14).[3]
Applications
It is often used in the lighting industry.[8]
References
- ^ "223646 Manganese(II) iodide 98%". Sigma-Aldrich. Retrieved 2011-08-05.
- ^ .
- ^ ISSN 0108-2701.
- S2CID 94812612.
- ^ ISBN 978-3-540-60035-0.
- ISBN 978-3-11-019060-1.
- .
- ^
Cepanec, Ivica (2004). Synthesis of Biaryls. Elseveir. p. 104. ISBN 0-08-044412-1. Retrieved 2008-06-18.

