Mazapertine
| |
| Names | |
|---|---|
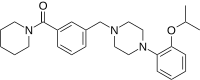
| Preferred IUPAC name
(Piperidin-1-yl){3-[(4-{2-[(propan-2-yl)oxy]phenyl}piperazin-1-yl)methyl]phenyl}methanone | |
| Other names
RWJ-37796
| |
| Identifiers | |
3D model (
JSmol ) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C26H35N3O2 | |
| Molar mass | 421.585 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Mazapertine (RWJ-37796) is an antipsychotic agent that was developed by Johnson & Johnson but never marketed. It exerts its pharmacological effect through affinity for dopamine D2, serotonin 5-HT1A, and α1-adrenergic receptors.[1]
Mazapertine is safe and well tolerated when administered orally.[2]
Analogs of mazapertine with conformational restriction have been prepared and have greater affinity for the 5-HT1A receptor.[3]
Synthesis
The laboratory synthesis of mazapertine has been reported.

