Transition metal alkyl complexes

Transition metal alkyl complexes are
Scope
Most metal alkyl complexes contain other, non-alkyl ligands. Great interest, mainly theoretical, has focused on the homoleptic complexes. Indeed, the first reported example of a complex containing a metal-sp3 carbon bond was the homoleptic complex diethylzinc. Other examples include hexamethyltungsten, tetramethyltitanium, and tetranorbornylcobalt.[3]

Structure of diethylzinc. The Zn-C bonds measure 194.8(5) pm, while the C-Zn-C angle is slightly bent with 176.2(4)°.[4]
Mixed ligand, or heteroleptic, complexes containing alkyls are numerous. In nature, vitamin B12 and its many derivatives contain reactive Co-alkyl bonds.

Hexamethyltungsten is an example of a "homoleptic" (all ligands being the same) metal alkyl complex.[3]
| example | comment |
|---|---|
| Ti(CH3)4 | only observed as monoetherate, d0[5] |
| [Ti(CH3)5]− | trigonal bipyramidal, d0[5] |
| [Ti2(CH3)9]− | one bridging methyl ligand, d0,d0[5] |
| [Zr(CH3)6]− | trigonal prismatic, d0[6][5] |
| [Hf(CH3)6]− | trigonal prismatic, d0[6] |
| [Nb(CH3)6]− | d0[3][6] |
| [Ta(CH3)6]− | d0[3] |
| Mo(CH3)5 | d1[7] |
| W(CH3)6 | trigonal prismatic, d0[3] |
| [Mn(CH3)4]2- | d5[8] |
| [Mn(CH3)6]2- | d3[9] |
| [Re(CH3)6] | d1[3] |
| [Fe(CH3)4]− | low-spin, d5, square planar[10] |
| [Co(CH3)4]− | square planar, d6[11] |
| [Rh(CH3)6]3- | d6[12] |
| [Ir(CH3)6]3- | d6[12] |
| [Ni(CH3)4]2- | d8[13] |
| [Pt(CH3)4]2-[12] | d8[14] |
| [Au(CH3)2]− | d10[15] |
| [Au(CH3)4]− | d8[15] |
| Zn(CH3)2 | d10 |
| Cd(CH3)2 | d10 |
| Hg(CH3)2 | d10 |
Preparation
Metal alkyl complexes are prepared generally by two pathways, use of alkyl nucleophiles and use of alkyl electrophiles. Nucleophilic sources of alkyl ligands include
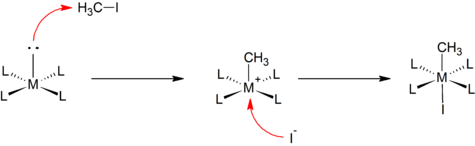
Electrophilic alkylation commonly starts with low valence metal complexes. Typical electrophilic reagents are
- CpFe(CO)2Na + CH3I → CpFe(CO)2CH3 + NaI
Many metal alkyls are prepared by oxidative addition:[2]
An example is the reaction of a
Agostic interactions and beta-hydride elimination
Some metal alkyls feature agostic interactions between a C-H bond on the alkyl group and the metal. Such interactions are especially common for complexes of early transition metals in their highest oxidation states.[18]
One determinant of the kinetic stability of metal-alkyl complexes is the presence of hydrogen at the position beta to the metal. If such hydrogens are present and if the metal center is
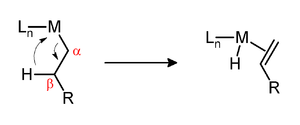
These conversions are assumed to proceed via the intermediacy of agostic interactions.
Catalysis
Many
References
- ISBN 978-3-527-29390-2.
- ^ ISBN 978-1-891389-53-5.
- ^ .
- PMID 21919175.
- ^ .
- ^ .
- doi:10.1039/b000987n.
- PMID 22148809.
- .
- PMID 25333789.
- S2CID 203144530.
- ^ .
- .
- .
- ^ .
- .
- .
- PMID 15054779.
- ISSN 0002-7863.