Metal dithiolene complex

Dithiolene metal complexes are
Dithiolene metal complexes have been studied since the 1960s when they were first popularized by Gerhard N. Schrauzer and Volker P. Mayweg, who prepared
The structural, spectroscopic, and electrochemical properties of many related complexes have been described.Structure
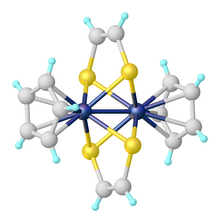
Dithiolene metal complexes can be found in coordination compounds where the metal centre is coordinated by one, two, or three dithiolene ligands. The tris(dithiolene) complexes were the first examples of trigonal prismatic geometry in coordination chemistry. One example is
Because of the unusual redox and intense optical properties of dithiolenes, the electronic structure of dithiolene complexes has been the subject of intense studies. 1,2-Dithiolene ligands can exist in three
Due to their delocalized electronic structure, 1,2-dithiolene complexes undergo reversible redox reaction. When oxidized, dithiolene complexes have greater 1,2-dithioketone character. In reduced complexes, the ligand assumes more ene-1,2-dithiolate character. These descriptions are evaluated by examination of differences in C-C and C-S bond distances. The true structure lies somewhere between these resonance structures. Reflecting the impossibility to provide an unequivocal description of the structure, McCleverty introduced the term 'dithiolene' to give a general name for the ligand that does not specify a particular oxidation state. This suggestion was generally accepted, and 'dithiolene' is now a universally accepted term. Only more recently the radical nature of monoanionic 1,2-dithiolene ligands has been pointed out.[7] While few examples of authentic dithiolene radicals have been reported, diamagnetism in neutral bis(1,2-dithiolene) complexes of divalent transition metal ions should be considered as a consequence of a string antiferromagnetic coupling between the two radical ligands.
- organyl.
Applications and occurrence
1,2-Dithiolene metal complexes occur widely in nature in the form of the molybdopterin-bound Mo and W-containing enzymes.
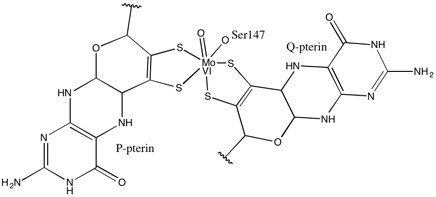
1,2-Dithiolene complexes applications are numerous, and span from superconductivity, to linear and non linear optics, to biochemistry. Commercial applications of 1,2-dithiolene complexes are limited. A few dithiolene complexes have been commercialized as dyes in laser applications (Q-switching, mode-locking). 1,2-Dithiolene complexes have been discussed in the context of
Preparation
From alkenedithiolates
Most dithiolene complexes are prepared by reaction of alkali metal salts of 1,2-alkenedithiolates with metal halides. A thiolate is the conjugate base of a thiol, so alkenedithiolate is, formally speaking, the conjugate base of an alkenedithiol. Common alkenedithiolates are 1,3-dithiole-2-thione-4,5-dithiolate[11] and maleonitriledithiolate (mnt2−):[12]
- Ni2+ + 2 (NC)2C2S2−2 → Ni[S2C2(CN)2]2−2
Some alkenedithiolates are generated in situ, often by complex organic reactions:
- cis-H2C2(SCH2Ph)2 + 4 Na → cis-H2C2(SNa)2 + 2 NaCH2Ph
Once generated, these anions are deployed as ligands:
- NiCl2 + 2 cis-H2C2(SNa)2 → Na2[Ni(S2C2H2)2] + 2 NaCl
Often the initially formed, electron-rich complex undergoes spontaneous air-oxidation:
- 2 [Ni(S2C2H2)2]2− + 4 H+ + O2 → 2 Ni(S2C2H2)2 + 2 H2O
From acyloins
An early and still powerful method for the synthesis of dithiolenes entails the reaction of α-hydroxyketones,
From dithietes
Although 1,2-dithiones are rare and thus not useful precursors, their valence isomer, the 1,2-dithietes are occasionally used. One of the more common dithiete is the distillable (CF3)2C2S2. This electrophilic reagent oxidatively adds to many low valent metals to give bis- and tris(dithiolene) complexes.
- Mo(CO)6 + 3 (CF3)2C2S2 → [(CF3)2C2S2]3Mo + 6 CO
- Ni(CO)4 + 2 (CF3)2C2S2 → [(CF3)2C2S2]2Ni + 4 CO
By reactions of metal sulfides with alkynes
Species of the type Ni[S2C2
History and nomenclature
Early studies on dithiolene ligands, although not called by that name until the 1960s,
References
- S2CID 248252909.
- ISBN 978-1-84973-624-4. Retrieved 2021-03-17.
- ISBN 978-0-471-37829-7.
- S2CID 34922450.
- .
- .
- ^ PMID 33185438.
- S2CID 85091949.
- PMID 11141557.
- PMID 16925411.
- .
- ISBN 978-0-470-13241-8.
- .
- ISBN 978-0-470-16611-6.
- ISBN 978-0-85404-366-8.
