Metallocene
A metallocene is a compound typically consisting of two
Some metallocenes consist of metal plus two cyclooctatetraenide anions (C
8H2−
8, abbreviated cot2−), namely the lanthanocenes and the actinocenes (uranocene and others).
Metallocenes are a subset of a broader class of compounds called sandwich compounds.[1] In the structure shown at right, the two pentagons are the cyclopentadienyl anions with circles inside them indicating they are aromatically stabilized. Here they are shown in a staggered conformation.
History
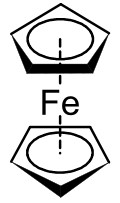
The first metallocene to be classified was
One of the very earliest commercial manufacturers of metallocenes was Arapahoe Chemicals in Boulder, Colorado[6]
Definition

The general name metallocene is derived from
In metallocene names, the prefix before the -ocene ending indicates what
In contrast to the more strict definition proposed by International Union of Pure and Applied Chemistry, which requires a d-block metal and a sandwich structure, the term metallocene and thus the denotation -ocene, is applied in the chemical literature also to non-transition metal compounds, such as barocene (Cp2Ba), or structures where the aromatic rings are not parallel, such as found in manganocene or titanocene dichloride (Cp2TiCl2).
Some metallocene complexes of actinides have been reported where there are three cyclopentadienyl ligands for a monometallic complex, all three of them bound η5.[7]
Classification
There are many (η5-C5H5)–metal complexes and they can be classified by the following formulas:[8]
| Formula | Description |
|---|---|
| [(η5-C5H5)2M] | Symmetrical, classical 'sandwich' structure |
| [(η5-C5H5)2MLx] | Bent or tilted Cp rings with additional ligands, L |
| [(η5-C5H5)MLx] | Only one Cp ligand with additional ligands, L ('piano-stool' structure) |
Metallocene complexes can also be classified by type:[8]
- Parallel
- Multi-decker
- Half-sandwich compound
- Bent metallocene or tilted
- More than two Cp ligands
Synthesis
Three main routes are normally employed in the formation of these types of compounds:[8]
Using a metal salt and cyclopentadienyl reagents
Sodium cyclopentadienide (NaCp) is the preferred reagent for these types of reactions. It is most easily obtained by the reaction of molten sodium and dicyclopentadiene.[9] Traditionally, the starting point is the cracking of dicyclopentadiene, the dimer of cyclopentadiene. Cyclopentadiene is deprotonated by strong bases or alkali metals.
- MCl2 + 2 NaC5H5 → (C5H5)2M + 2 NaCl (M = V, Cr, Mn, Fe, Co; solvent = THF, DME, NH3)
- CrCl3 + 3 NaC5H5 → [(C5H5)2Cr] + 1⁄2 "C10H10" + 3 NaCl
NaCp acts as a reducing agent and a ligand in this reaction.
Using a metal and cyclopentadiene
This technique provides using metal atoms in the gas phase rather than the solid metal. The highly reactive atoms or molecules are generated at a high temperature under vacuum and brought together with chosen reactants on a cold surface.
- M + C5H6 → MC5H5 + 1⁄2 H2 (M = Li, Na, K)
- M + 2 C5H6 → [(C5H5)2M] + H2 (M = Mg, Fe)
Using cyclopentadienyl reagents
A variety of reagents have been developed that transfer Cp to metals. Once popular was
Many other methods have been developed.
- Cr(CO)6 + 2 C5H6 → Cr(C5H5)2 + 6 CO + H2
Metallocenes generally have high thermal stability. Ferrocene can be sublimed in air at over 100 °C with no decomposition; metallocenes are generally purified in the laboratory by vacuum
Structure
A structural trend for the series MCp2 involves the variation of the M-C bonds, which elongate as the valence electron count deviates from 18.[11]
| M(C5H5)2 | rM–C (pm) | Valence electron count |
|---|---|---|
| Fe | 203.3 | 18 |
| Co | 209.6 | 19 |
| Cr | 215.1 | 16 |
| Ni | 218.5 | 20 |
| V | 226 | 15 |
In metallocenes of the type (C5R5)2M, the cyclopentadienyl rings rotate with very low barriers. Single crystal
Spectroscopic properties[8]
Vibrational (infrared and Raman) spectroscopy of metallocenes
Infrared and Raman spectroscopies have proved to be important in the analysis of cyclic polyenyl metal sandwich species, with particular use in elucidating covalent or ionic M–ring bonds and distinguishing between central and coordinated rings. Some typical spectral bands and assignments of iron group metallocenes are shown in the following table:[8]
| Ferrocene (cm−1) | Ruthenocene (cm−1) | Osmocene (cm−1) | |
|---|---|---|---|
| C–H stretch | 3085 | 3100 | 3095 |
| C–C stretch | 1411 | 1413 | 1405 |
| Ring deformation | 1108 | 1103 | 1096 |
| C–H deformation | 1002 | 1002 | 995 |
| C–H out-of-plane bend | 811 | 806 | 819 |
| Ring tilt | 492 | 528 | 428 |
| M–ring stretch | 478 | 446 | 353 |
| M–ring bend | 170 | 185 | – |
NMR (1H and 13C) spectroscopy of metallocenes
Nuclear magnetic resonance (NMR) is the most applied tool in the study of metal sandwich compounds and organometallic species, giving information on nuclear structures in solution, as liquids, gases, and in the solid state. 1H NMR chemical shifts for paramagnetic organotransition-metal compounds is usually observed between 25 and 40 ppm, but this range is much more narrow for diamagnetic metallocene complexes, with chemical shifts usually observed between 3 and 7 ppm.[8]
Mass spectrometry of metallocenes
Mass spectrometry of metallocene complexes has been very well studied and the effect of the metal on the fragmentation of the organic moiety has received considerable attention and the identification of metal-containing fragments is often facilitated by the isotope distribution of the metal. The three major fragments observed in mass spectrometry are the molecular ion peak, [C10H10M]+, and fragment ions, [C5H5M]+ and M+.
Derivatives
After the discovery of ferrocene, the synthesis and characterization of derivatives of metallocene and other sandwich compounds attracted researchers’ interests.
Metallocenophanes
Polynuclear and heterobimetallic metallocenes
- Ferrocene derivatives: biferrocenophanes have been studied for their mixed delocalized.
- Ruthenocene derivatives: in the solid state biruthenocene is disordered and adopts the transoid conformation with the mutual orientation of Cp rings depending on the intermolecular interactions.
- dimerize immediately at room temperature and they have been observed in matrix isolation.
Multi-decker sandwich compounds

Triple-decker complexes are composed of three Cp anions and two metal cations in alternating order. The first triple-decker sandwich complex, [Ni2Cp3]+, was reported in 1972.[12] Many examples have been reported subsequently, often with boron-containing rings.[13]
Metallocenium ions
The most famous example is
Applications
Many derivatives of early metal metallocenes are active catalysts for
Potential applications
The ferrocene/
Metallocene dihalides [Cp2MX2] (M = Ti, Mo, Nb) exhibit anti-tumor properties, although none have proceeded far in clinical trials.[15]
See also
- Jemmis mno rules
- Actinocenes
- f-block metallocene
References
- ^ .
- ^ S2CID 4181383.
- ^ .
- .
- .
- ISSN 0003-2700.
- .
- ^ ISBN 978-0632041626.
- .
- S2CID 209650632.
- . Discusses all metallocene structures available at that time.
- ISSN 0094-5714.
- .
- .
- .
Additional references
- Salzer, A. (1999). "Nomenclature of Organometallic Compounds of the Transition Elements". S2CID 14367196.
- ISBN 0470257628
- Miessler, Gary L.; Tarr, Donald A. (2004). Inorganic Chemistry. Upper Saddle River, NJ: Pearson Education. ISBN 978-0-13-035471-6.
- Cotton, F. A.; Wilkinson, G. (1988). Inorganic Chemistry (5th ed.). Wiley. pp. 626–7.[ISBN missing]
- Togni, A.; Halterman, R. L. (1998). Metallocenes. Wiley-VCH.[ISBN missing]
