User:Epipelagic/sandbox/current2
| This is a Wikipedia user page. This is not an encyclopedia article or the talk page for an encyclopedia article. If you find this page on any site other than Wikipedia, you are viewing a mirror site. Be aware that the page may be outdated and that the user in whose space this page is located may have no personal affiliation with any site other than Wikipedia. The original page is located at https://en.wikipedia.org/wiki/User:Epipelagic/sandbox/current2. |
RESOURCES AND WORKING DRAFTS ONLY
References for processing

- ISBN 9781774200285.
- S2CID 1382541.)
{{cite journal}}: CS1 maint: unflagged free DOI (link - S2CID 208334090.)
{{cite journal}}: CS1 maint: unflagged free DOI (link - PMID 29780377.)
{{cite journal}}: CS1 maint: unflagged free DOI (link - PMID 30733706..
{{cite journal}}: CS1 maint: unflagged free DOI (link) Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License - S2CID 218973418.)
{{cite journal}}: CS1 maint: unflagged free DOI (link - doi:10.5194/cp-15-1171-2019.)
{{cite journal}}: CS1 maint: unflagged free DOI (link
References
Aeroplankton
Biogenic aerosols
Aerosols affect cloud formation, thereby influencing sunlight irradiation and precipitation, but the extent to which and the manner in which they influence climate remains uncertain.[1] Marine aerosols consist of a complex mixture of sea salt, non-sea-salt sulfate and organic molecules and can function as nuclei for cloud condensation, influencing the radiation balance and, hence, climate.[2][3] For example, biogenic aerosols in remote marine environments (for example, the Southern Ocean) can increase the number and size of cloud droplets, having similar effects on climate as aerosols in highly polluted regions.[3][4][5][6] Specifically, phytoplankton emit dimethylsulfide, and its derivate sulfate promotes cloud condensation.[2][7] Understanding the ways in which marine phytoplankton contribute to aerosols will allow better predictions of how changing ocean conditions will affect clouds and feed back on climate.[7] In addition, the atmosphere itself contains ~1022 microbial cells, and determining the ability of atmospheric microorganisms to grow and form aggregates will be valuable for assessing their influence on climate.[8][9]
Aeroplankton as extremophiles
Extremophile organisms capable of growing in extreme conditions draw considerable attention since they show that life is robust and adaptable and help us understand its limits. In addition, they show a high biotechnological potential [1, 2]. Most of the best-characterized extreme environments on Earth are geophysical constraints (temperature, pressure, ionic strength, radiation, etc.) in which opportunistic microorganisms have developed various adaptation strategies. Deep-sea environments, hot springs and geysers, extreme acid waters, hypersaline environments, deserts, and permafrost or ice are some or the most recurrent examples of extreme environments [3]. However, the atmosphere is rarely thought of as an extreme habitat. In the atmosphere, the dynamics of chemical and biological interactions are very complex, and the organisms that survive in this environment must tolerate high levels of UV radiation, desiccation (wind drying), temperature (extremely low and high temperatures), and atmospheric chemistry (humidity, oxygen radicals, etc.) [4]. These factors turn the atmosphere (especially its higher layers) into one of the most extreme environments described to date and the airborne microorganisms into extremophiles or, at least, multiresistant ones [5].[10]
It is known that airborne cells can maintain viability during their atmospheric residence and can exist in the air as spores or as vegetative cells thanks to diverse molecular mechanisms of resistance and adaptation [2, 6]. The big question is whether some of them can be metabolically active and divide. Bacterial residence times can be several days, which facilitate transport over long distances. This fact, together with the extreme conditions of the atmosphere, has led researchers to think for years that they do not remain active during their dispersion. However, recent studies strongly suggest that atmospheric microbes are metabolically active and were aerosolized organic matter and water in clouds would provide the right environment for metabolic activity to take place. Thus, the role played by microorganisms in the air would not only be passive but could also influence the chemistry of the atmosphere. In any case, only a certain fraction of bacteria in the atmosphere would be metabolically active [2, 7].[10]
Despite recognizing its ecological importance, the diversity of airborne microorganisms remains largely unknown as well as the factors influencing diversity levels. Recent studies on airborne microbial biodiversity have reported a diverse assemblage of bacteria and fungi [4, 8, 9, 10, 11, 12], including taxa also commonly found on leaf surfaces [13, 14] and in soil habitats [15]. The abundance and composition of airborne microbial communities are variable across time and space [11, 16, 17, 18, 19]. However, the atmospheric conditions responsible for driving the observed changes in microbial abundances have not been thoroughly established. One reason for these limitations in the knowledge of aerobiology is that until recently, microbiological methods based on culture have been the standard, and it is known that such methods capture only a small portion of the total microbial diversity [20]. In addition, because pure cultures of microorganisms contain a unique type of microbes, culture-based approaches miss the opportunity to study the interactions between different microbes and their environment.[10]
Another limitation for the study of aerial microbial ecology at higher altitudes or in open ocean areas is the difficulty of repeated and dedicated use of airborne platforms (i.e., aircraft or balloons) to sample the air. Most studies to date on the atmospheric microbiome are restricted to samples collected near the Earth’s surface (e.g., top of mountains or buildings). Aircraft, unmanned aerial systems (UASs), balloons or even rockets, and satellites could represent the future in aerobiology knowledge [5, 21, 22]. These platforms could open the door to conducting microbial studies in the stratosphere and troposphere at high altitudes and in open-air masses, where long-range atmospheric transport is more efficient, something that is still poorly characterized today. The main challenge in conducting these kinds of studies stems from the fact that microbial collection systems are not sufficiently developed. There is a need for improvement and implementation of suitable sampling systems for platforms capable of sampling large volumes of air for subsequent analyses using multiple techniques, as this would provide a wide range of applications in the atmospheric, environmental, and health sciences.[10]
In aerobiology, dust storms deserve special mention. Most of them originate in the world’s deserts and semideserts and play an integral role in the Earth system [23, 24]. They are the result of turbulent winds, including convective haboobs [25]. This dust reaches concentrations in excess of 6000 μg m−3 in severe events [26]. Dust and dust-associated bacteria, fungal spores, and pollen can be transported thousands of kilometers in the presence of dust [9].[10]
References
- S2CID 58612273.
- ^ S2CID 4321239.
- ^ doi:10.5194/acp-13-3979-2013.)
{{cite journal}}: CS1 maint: unflagged free DOI (link - S2CID 36030601.
- .
- doi:10.5194/acp-13-4235-2013.)
{{cite journal}}: CS1 maint: unflagged free DOI (link - ^ PMID 29459666.
- S2CID 61155774.
- ^ a b c d e Cite error: The named reference
Aguilera2018was invoked but never defined (see the help page).
Lanternfish

The vast darkness of the deep sea is an environment with few obvious genetic isolating barriers, and little is known regarding the macroevolutionary processes that have shaped present-day biodiversity in this habitat. Bioluminescence, the production and emission of light from a living organism through a chemical reaction, is thought to occur in approximately 80 % of the eukaryotic life that inhabits the deep sea (water depth greater than 200 m). In this study, we show, for the first time, that deep-sea fishes that possess species-specific bioluminescent structures (e.g., lanternfishes, dragonfishes) are diversifying into new species at a more rapid rate than deep-sea fishes that utilize bioluminescence in ways that would not promote isolation of populations (e.g., camouflage, predation). This work adds to our understanding of how life thrives and evolution shaped present-day biodiversity in the deep sea, the largest and arguably least explored habitat on earth.[1]
Bioluminescence is the final product of a biochemical reaction whereby energy is converted to light following the breakdown of molecular bonds, typically the molecular decomposition of luciferin substrates by the enzyme luciferase in the presence of oxygen (Herring 1987; Haddock et al. 2010; Widder 2010). Bioluminescence has repeatedly evolved across the tree of life, from single-celled bacteria and dinoflagellates to fungi, jellyfishes, insects, and vertebrates (Herring 1987; Haddock et al. 2010; Widder 2010). Among animals, bioluminescence is used to communicate, defend against predation, and find or attract prey (Herring 1987; Haddock et al. 2010; Widder 2010). In some cases (e.g., fireflies, ostracods), unique bioluminescent signals have been hypothesized to aid in the process of speciation, with species recognition providing a mechanism to promote reproductive isolation among populations (Palumbi 1994; Branham and Greenfield 1996). In these bioluminescent organisms, the animals broadcast their identity with distinct light patterns. Among vertebrate lineages, bioluminescence has evolved only in cartilaginous and bony fishes that inhabit marine environments, with more than 80 % of luminous vertebrates confined to the deep sea (Herring 1987; Haddock et al. 2010).[1]
Within teleosts, the production and emission of light is predominantly generated endogenously (e.g., the photophores of hatchetfishes and lanternfishes; Herring 1987; Haddock et al. 2010; Kronstrom and Mallefet 2010; Widder 2010), or through bacterially-mediated symbiosis (e.g., most anglerfish lures, flashlightfish subocular organs; Herring 1987; Dunlap et al. 2007; Haddock et al. 2010; Widder 2010). The functional utility of bioluminescence in a marine environment is both fascinating and wildly diverse, with incredible morphological specializations ranging from elongate species-specific barbels and lures to complex arrangements of photophores that are used to aid camouflage, defense, predation, and communication (Herring 1987; Haddock et al. 2010; Widder 2010). The utility of luminescence for predation in fishes ranges from red searchlights in the loosejaw dragonfishes Malacosteus (Douglas et al. 1998), to modified dorsal-fin spines in ceratioid anglerfishes that are used to lure in unsuspecting prey (Herring 2007). Camouflage and defensive strategies have repeatedly evolved across deep-sea marine lineages, including ventral counter-illumination, whereby an organism utilizes their bioluminescent photophores to match the intensity of downwelling light in an attempt to hide their silhouette from predators lurking below (Hastings 1971). Some bioluminescent organisms are even hypothesized to utilize light for intraspecific communication, including sexual selection (ponyfishes; Sparks et al. 2005; Chakrabarty et al. 2011) and species recognition (fireflies; Branham and Greenfield 1996).[1]
The open ocean is an environment with few reproductive isolating barriers (Palumbi 1994) and comparatively low levels of species richness across different marine environments. Low species diversity in this environment is attributed to a general lack of physical barriers to gene flow and broad sympatry of populations (Palumbi 1994). Few studies have investigated patterns and mechanisms of diversification in the deep sea, and long-term behavioral studies are limited by the extreme conditions of the habitat (e.g., pressure, temperature). Lanternfishes (Myctophidae) are among the most species-rich families of marine teleosts and are endemic to this deep-sea, open-ocean environment (Eschmeyer 2013). Lanternfishes are consistently among the most abundant marine vertebrates collected around the globe in mid- to deep-water trawls (Sutton et al. 2010). The other predominant bioluminescent teleosts in this environment are the bristlemouths (Gonostomatidae: Cyclothone, Gonostoma, and Sigmops), which are estimated to be the most abundant vertebrates on earth (Sutton et al. 2010). Other common mesopelagic taxa include the marine hatchetfishes (Sternoptychidae) and dragonfishes (Stomiidae) (Sutton et al. 2010). Whereas bristlemouths (Gonostomatidae), overwhelmingly Cyclothone, have a strikingly higher biomass and account for more than 50 % of the total vertebrate abundance in mid-water habitats (~100–1,000 m), lanternfishes (Myctophidae) are the second most abundant family in this realm (Sutton et al. 2010). Interestingly, lanternfishes have diversified into 252 species, whereas only 21 species of bristlemouths have been described worldwide (Eschmeyer 2013). Bristlemouths and lanternfishes are superficially similar in size and body plan, with both groups possessing ventrally oriented bioluminescent photophores that provide camouflage, via ventral counter-illumination, against predation. However, lanternfishes have evolved an incredible array of modifications to their light-organ systems (photophores laterally on the body, sexually dimorphic luminescent organs on the tail or head) (Paxton 1972) that may have a significant impact on the evolutionary history and diversification of these fishes.[1]
History of biogeochemistry
Introduction

Born from multidisciplinary interactions between biological, geological, and chemical sciences early in the 19–20th centuries, biogeochemistry has continued to expand its scope in the 21st century on scales that range from
Disparate beginnings in the age of enlightenment: 17–19th centuries
Perhaps the earliest example of linking organic and inorganic substances with large earthly cycles, the rudiments of biogeochemistry, can in part, be traced to Empedocles (483–424 B.C.), who divided the physical universe into air, water, fire, and earth, as well as a disciple of Confucius (551–479 B.C.), who developed the five universal element system.[10][11][12] However, as Gorham (1991) explains, it was not until the period between the 17th and 19th centuries, that we begin to see studies of photosynthesis (e.g. Plattes 1639; Hooke 1687; Priestley 1772), organic matter decomposition;[13][14][15][16] metabolism (e.g. Davy 1813; Leibig 1840; Forschhammer 1865), plant nutrition (e.g. Digby 1669; Leibig 1855; Salm-Horstmar, 1856) and weathering (e.g. Home 1757; Hutton 1795; Thaer 1810; Bischof 1854), that really begins to set the stage for the emergence of biogeochemical concepts. For example, some of the first chemical budgets and descriptions of elemental cycles linked soil fertility (e.g. Plattes 1639; Lawes and Gilbert 1882; Dumas 1841) and plant transpiration to the hydrologic cycle (e.g., Halley 1687; Woodward 1699). In fact, Gorham (1991) posits such studies were key in the establishment of the: (1) pathways and key linkages of inorganic and organic substance processing with the hydrologic cycle; (2) importance of carbonic acid, generated via metabolic pathways, in weathering; (3) foundations in plant nutrition; (4) importance of microbes in organic matter decay and elemental cycles; and (5) recognition of elemental cycles on a global basis. From this early work, we see the conceptual emergence of the term biosphere, first used by Lamarck (1802) and more formally developed by Suess (1875). This concept was later adopted by Arrhenius (1896), who began to make important linkages between geochemistry and the biosphere. However, the true concept of biosphere, as we think of it today, was not realized until Vernadsky (1926) (Fig. 1)—as further promulgated by George Evelyn Hutchinson (1903–1991). Hutchinson (1970) writes that: “The concept played little part in scientific thought, however, until the publication, first in Russian in 1926 (actually 1924) and later in French in 1929 (under the title La Biosphere), of two lectures by the Russian mineralogist Vladimir Ivanovitch Vernadsky. It is essentially Vernadsky's concept of the biosphere, developed about 50 years after Suess wrote, that we accept today. Vernadsky considered that the idea ultimately was derived from the French naturalist Jean Baptiste Lamarck, whose geochemistry, although archaically expressed, was often quite penetrating.” The boundaries between these different physical states of rock, gas, water, and organic matter (Fig. 1) change when phase transitions are thermodynamically in favor of a molecule that is catalyzed into transition to another state; understanding the controls of these transitions in the biosphere is central to biogeochemistry (see Degens 1989).[3]
References
- ^ doi:10.1007/s00227-014-2406-x.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License - doi:10.1007/BF00002942.)
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help - ^ doi:10.1007/s10533-020-00708-0..
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License - doi:10.1890/03-0242.)
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help - OCLC 37211183.
- ^ doi:10.1038/s43017-019-0005-6.)
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help - ^ Mayr E. (1961) "Cause and effect in biology". Science, 134(3489): 1501-6.
- ^ Simpson GG (1967) "The crisis in biology". Am Scholar, 36(3): 363–377
- doi:10.1007/s100219900025.)
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help - ^ Browne CA (1944) A Source Book of Agricultural Chemistry. The Chronica Botanica Co.
- OCLC 1082196098.
- OCLC 851751337.
- ^ MacBride D (1674) Experimental essays. A Miller, London.
- ^ Jameson R (1800) "On peat or turf". Trans Dublin Society, 1: 10.
- ^ Schwann T (1837) "Vorlaufige Mittheilung, betreffend Versuche iiber die Weingahrung und Faulnis". Annalen der Physik und Chemie, 41:184–193. Seen in translation by Brock (1961)/
- ^ Cohn FJ (1872) Uber Bakterien, die Kleinsten Leben den Wesen. Seen in translation by Dolley CS (1881), published 1939. Johns Hopkins Press, Biol Biochem, 94:200–210.
Neuston





The
Organisms that live on the ocean’s surface—termed neuston (or sometimes pleuston)—are adapted to survive in this razor-thin environment. Concentrated neustonic life is the foundation of the Sargasso Sea: a floating ecosystem in the North Atlantic subtropical gyre. The Sargasso Sea is named after the floating neustonic Sargassum algae.[2][3] and serves as a haven for biodiversity in the open ocean. The Sargasso Sea is also a nursery ground for a variety of threatened and endangered organisms.[2][1]
Neustonic animals likely play an important role in the ecology of the Sargasso Sea. Neustonic animals include a variety of
A wide variety of predators rely on neustonic animals. Neuston are important prey for turtles,[4][5][6] diverse seabirds,[7] and a variety of fish species.[1] Neuston in turn consume pelagic prey like copepods, fish eggs and larvae, and gelatinous zooplankton.[8][9][10] And pelagic life cycle stages of some neustonic animals may further trophically link the surface to deeper water.[1] However, we know very little about the biology of neustonic animals in general or in the Sargasso Sea in particular. This makes it difficult to assess their role in the health of the Sargasso Sea ecosystem. This is especially true now, as massive amounts of floating plastic become concentrated in this area, occupying the same surface layer where neuston live.[11][12][13][14][1]
In March 2020, the author examined the natural history, including feeding biology, reproductive biology, and behavior, of neustonic animals in the Sargasso Sea (Fig. 1). The author observed species-specific predator-prey interactions, high rates of reproduction that may have evolved to hedge against patchy resources, and unique behaviors producing ephemeral habitat at the surface.[1]
Dietary niche separation
Floating marine organisms occupy an incredibly narrow habitat range with little control over their direction of movement; for most species, prey is not pursued but encountered haphazardly. Dietary specialization of closely related species may allow different species to co-occur in this environment without competition (Bieri 1966, 1970). This is the first study to show evidence for niche partitioning of neustonic mollusks in the Sargasso Sea. Despite co-occurring in the same region, J. pallida and J. janthina appear to prefer different prey. While J. janthina preyed upon Physalia sp. and V. velella, J. pallida appears behaviorally adapted to prey on V. velella and did not prey upon Physalia sp. These results are similar to those by Bieri (1966) who observed that J. prolongata in the eastern North Pacific prefer V. velella and P. porpita but do not prey upon Physalia sp.[1]
For both G. atlanticus and Janthina spp., consuming only part of a prey item was more common than full consumption. Both J. pallida and J. janthina preyed largely on the margins of their prey, which is consistent with observations by Bieri for J. prolongata (Bieri 1966). While G. atlanticus preyed upon both species of cnidarians, unlike J. pallida and J. janthina, it also often crawled under prey to feed on central zooids. Bieri (1966) also observed Glaucus much more actively feeding on gastrozooids compared to margin tissue.[1]
Differences between Janthina and Glaucus in the region of prey on which they feed may create further niche partitioning. This difference may be due to differences in the floating mechanism or prey defense. Glaucus atlanticus holds swallowed air in its stomach (Lalli and Gilmer 1989), while Janthids build bubble rafts and cannot swim (Wilson 1956). This makes crawling under prey considerably riskier for Janthids. Differences in the prey region consumed may also be due to defenses by V. velella and Physalia. After being attacked by J. pallida, V. velella appeared to produce a mysterious antipredator barrier that prevented J. pallida from getting close again. While this may inhibit Janthids from prolonged feeding, G. atlanticus can “grab” onto prey using cerata, potentially rendering these barriers less effective. For these reasons (and likely others), G. atlanticus is able to take advantage of central zooids that it must crawl underneath to eat, while Janthids may be more likely to prey upon the margins. However, these results should also be considered in light of possible ontogenetic shifts. Small Janthinds have been reported to crawl on much larger prey and eat below the margin (Bieri 1966). If they disturb their prey’s float or are too heavy for their prey, both will sink. Further work should examine how predation changes over development and across species of different sizes.[1]
Reproduction and implications for connectivity and dispersal
The surface habitat is distinct from both pelagic and benthic environments, sharing qualities of each but not fully analogous to either. The surface is a barrier, similar to the benthos, but less rigid and with no fixed position. For this reason, animals at the surface likely evolved unique life histories distinct from both benthic and pelagic species. In this study, the reproduction of three neustonic species was examined. Both J. pallida and G. atlanticus produced large numbers of embryos (though only the rate of G. atlanticus was measured), and V. velella produced variable numbers of medusae depending on conditions.[1]
All three species observed here have nonmobile surface stages at the mercy of currents, waves, and weather. These species may be aggregated into patchy distributions or spread over large distances. This is a remarkable challenge specifically for predators J. pallida and G. atlanticus, which cannot seek out prey. A high number of larvae for J. pallida and G. atlanticus suggests a form of reproductive bet-hedging against these uncertain conditions. In fact, G. atlanticus has high fecundity compared even to similarly sized benthic eolid nudibranchs (Ross and American 1990). Perhaps unsurprisingly, the rate of embryo production in G. atlanticus is dependent on food availability: after several days without food embryo production decreases (Ross and American 1990).[1]
Similar to the species observed here, the European eel (Anguilla anguilla), which migrates to spawn in the Sargasso Sea, also has an extremely high fecundity rate, with millions of eggs being produced by a single eel female (MacNamara and McCarthy 2012). However, this species is still in precipitous decline and listed as critically endangered. High reproduction rates of Sargasso Sea organisms may provide some bet-hedging in undisturbed systems, but they may still be vulnerable to human impacts.[1]
According to Ross and American (1990), embryonic development of G. atlanticus takes about 3 days at 19 °C, and larvae have been kept for over a week in captivity before eventually dying without metamorphosis. Their healthy development at 19 °C is a clue to their oceanic habitat: if G. atlanticus embryos sank below the thermocline before completing embryogenesis, development may require cooler conditions. However, tolerance to sea surface temperatures suggests they can, at least, remain relatively shallow for an extended period. This may also be true for V. Velella. Some studies have reported V. velella medusae and larvae in deep water (however, they used a net that remained open while descending and ascending (Woltereck 1904)), while others have found medusae near the surface and propose an epipelagic distribution (Larson 1980). The negative gravitropism of several-day-old medusae, observed here, suggests they do occupy the epipelagic, at least during some times of the day or during specific stages of development. This is consistent with the presence of symbiotic zooxanthellae in medusae (Larson 1980), which could provide nutrients in prey-depleted oligotrophic waters. In fact, the mouths of young medusae may not fully develop for several days after liberation (Brinckmann-Voss 1970), resulting in young medusae being reliant on the zooxanthellae for sustenance. Future studies should look for evidence of changes in gravitropism throughout a 24-h cycle and at different ages, as well as the presence of medusae and larvae in samples from different depths.[1]
Janthina pallida larvae are poorly known. Embryos of most Janthids remain affixed to the float through development, tying their fate to ocean surface conditions. However, the potential positive gravitropism of newly hatched veligers observed here suggests they may retreat into deeper water (though perhaps still epipelagic). However, a more focused study of this should be conducted to confirm. Regardless of their larval habitat in the pelagic zone, at some point during development, these planktotrophic larvae return to the surface, possibly aided by a small secreted mucous net or string of bubbles (Wilson 1956; Lalli and Gilmer 1989).[1]
Very little is known about the temporal and spatial dynamics of floating neustonic animals. Some seasonality has been suggested for V. velella off the California coast (Bieri 1977), though this may also (or instead) be due to seasonal changes in wind or currents. If neustonic species do exhibit seasonality, presumably, V. velella and Physalia sp. would recruit ahead of or concurrent with their predators J. pallida and G. atlanticus, unless some or all species persist across seasons. And V. velella and J. pallida must be present before any organism that might utilize their floating substrates can proliferate (see section “Habitat construction”).[1]
Habitat construction
Janthina snails may appreciably contribute to floating temporary habitats in the open ocean. The rapid rate of new raft construction and the abandonment of floating unoccupied rafts could aid in larval dispersal and generate substrate for rafting organisms or
In addition to floating Janthid rafts, the skeletal floats of V. Velella are also buoyant, and neustonic and rafting barnacles settle on them. Although most V. velella were not fully consumed, a single G. atlanticus cleared a small V. velella overnight, with only the floating skeleton remaining. High lethal predation on V. velella may create substrates for additional rafting organisms and neuston to use.[1]
We know very little about the biology and ecology of surface marine life. This study presents new information about the basic biology, predation, reproduction, and behavior of neustonic animal species in the Sargasso Sea.[1]
The Sargasso Sea is a critical habitat in the North Atlantic, including for commercial and endemic species, juvenile turtles, endangered eels, and countless other organisms that utilize this habitat at various times in their life history. Understanding the biology and ecology of neustonic species is critical to our understanding of broader ocean connectivity and food web dynamics. This study suggests that predatory niche partitioning, high fecundity rates, and habitat construction may be important features of neustonic animal biology.[1]
References
- ^ doi:10.1007/s12526-021-01233-5.).
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License Cite error: The named reference "Helm2021" was defined multiple times with different content (see the help page
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License Cite error: The named reference "Helm2021" was defined multiple times with different content (see the help page - ^ a b Trott TM, Mckenna SA, Pitt JM et al (2011) E "Efforts to enhance protection of the Sargasso Sea". Proceedings of the 63rd Gulf and Caribbean Fisheries Institute, 282–288.
- ^ Pendleton L, Krowicki F, Strosser P, Hallett-Murdoch J (2014) "Assessing the economic contribution of marine and coastal ecosystem services in the Sargasso Sea". Nicholas Institute for Environmental Policy Solutions, Duke University
- doi:10.1007/s00227-001-0737-x.)
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help - ^ Parker DM, Cooke WJ, Bulletin GBF, 2005 (2003) "Diet of oceanic loggerhead sea turtles (Caretta caretta) in the central North Pacific". Fish Bull, Natl Ocean Atmos Adm, 103: 142–152.
- doi:10.1007/s00227-015-2738-1.)
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help - ^ Harrison CS, Hida TS, Seki MP (1983) ""Hawaiian seabird feeding ecology"". Wildlife Monograph, 85: 3–71
- ^ Bieri R (1961) "Post-larval food of the pelagic coelenterate, Velella lata", Pac Sci, 15: 553–556
- ^ Purcell JE (1984) "Predation on larval fish by Portuguese man of war, Physalia physalis". Mar Ecol Prog Ser, 19: 189–191
- doi:10.1007/s10750-012-1052-x.)
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help - doi:10.1126/science.175.4027.1240.)
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help - doi:10.1016/0025-326X(82)90038-8.)
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help - ^ Siuda A (2011) "Summary of Sea Education Association", Long-term Sargasso Sea Surface Net Data.
- doi:10.1007/s00227-014-2539-y.)
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help
Portuguese man o' war
-
Looking down from above a man o' war, showing its sail. Sails can be left-handed or right-handed.
-
A typical square-rigged caravel (Livro das Armadas)]]
References
Zooplankton vertical migration
- Bandara, Kanchana; Varpe, Øystein; Wijewardene, Lishani; Tverberg, Vigdis; Eiane, Ketil (2021-05-04). "Two hundred years of zooplankton vertical migration research". Biological Reviews. 96 (4). Wiley: 1547–1589. ISSN 1464-7931..
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License
Spermbot

Symbioses of cyanobacteria
- in marine environments
Cyanobacteria are a diversified phylum of nitrogen-fixing, photo-oxygenic bacteria able to colonize a wide array of environments. In addition to their fundamental role as diazotrophs, they produce a plethora of bioactive molecules, often as secondary metabolites, exhibiting various biological and ecological functions to be further investigated. Among all the identified species, cyanobacteria are capable to embrace symbiotic relationships in marine environments with organisms such as protozoans, macroalgae, seagrasses, and sponges, up to ascidians and other invertebrates. These symbioses have been demonstrated to dramatically change the cyanobacteria physiology, inducing the production of usually unexpressed bioactive molecules. Indeed, metabolic changes in cyanobacteria engaged in a symbiotic relationship are triggered by an exchange of infochemicals and activate silenced pathways. Drug discovery studies demonstrated that those molecules have interesting biotechnological perspectives. In this review, we explore the cyanobacterial symbioses in marine environments, considering them not only as diazotrophs but taking into consideration exchanges of infochemicals as well and emphasizing both the chemical ecology of relationship and the candidate biotechnological value for pharmaceutical and nutraceutical applications.[2]
Cyanobacteria are a wide and diversified phylum of bacteria capable of photosynthesis. They are found in symbiosis with a remarkable variety of hosts, in a wide range of environments (Figure 1). Symbiotic relationships concern advantages and disadvantages for the organisms involved. Symbiosis, indeed, can be advantageous for only one of the involved organisms (commensalism, parasitism), or for both (mutualism) [1]. Symbiotic interactions are widespread and involve organisms among life domains, in both Eukaryota and Prokaryota (Archaea and Bacteria). Among prokaryotes, various species have been demonstrated to be associated with invertebrates such as sponges [2,3], corals [4,5,6,7], sea urchins [8], ascidians [9,10], and mollusks [11,12,13]. In addition, symbiotic relationships between bacteria and various microorganisms such as Retaria [14,15], Myzozoa [16], Ciliophora, and Bacillariophyceae [17] were investigated in the frame of the peculiar N2 fixing process performed by various associated prokaryotes. In fact, cyanobacteria are able to perform nitrogen fixation and, among all the symbiotic interactions they are able to establish, the nitrogenase products represent the major contribution to the partnership [18]. Nitrogen-fixing organisms are often called diazotrophs and their diazotroph-derived nitrogen (DDN) gives their hosts the advantage to populate nitrogen-limited environments [19,20]. Cyanobacterial symbionts (also named cyanobionts) are active producers of secondary metabolites and toxins [21], able to synthesize a large array of bioactive molecules, such as photoprotective and anti-grazing compounds [4,22]. In addition, cyanobionts have the advantage to be protected from environmental extreme conditions and from predation/grazing. In parallel, hosting organisms grant enough space to cyanobionts for growing at low competition levels. Several investigations demonstrated an influence of host organisms on the production of cyanobiont secondary metabolites, as in the case of the symbiotic interaction of Nostoc cyanobacteria with the terrestrial plant of Gunnera and Blasia genera [23]. Indeed, changes in the expression of secondary metabolites, as in the cases of the cyanobacterial nostopeptolide synthetase gene and the altered secretion of various nostopeptolide variants, were recorded in Nostoc punctiforme according to the presence of the host [24]. Changes in the metabolic profiles have probably a clear role in the formation of cyanobacterial motile filaments (hormogonia) and, most probably, they affect the infection process and the symbiotic relationship itself [24]. This suggests that cyanobacterial secondary metabolites may play a key role in host–cyanobacterium communications.[2]
There are lines of evidence that cyanobionts produce novel compounds of interest to pharmaceutical research [25,26], exhibiting cytotoxic and antibacterial activities. Some of these molecules are produced by cyanobacteria only in a symbiotic relationship, as in the case of polyketide nosperin (Figure 2) [27].[2]
Cyanobacteria are capable of establishing various types of symbiosis, with variable degrees of integration with the host, and probably symbiosis emerged independently with peculiar characteristics [28,29,30]. Symbionts are transferred to their hosts by a combination of vertical and horizontal transmission, with some strains passed down from ancestral lineage, while others are acquired by the surrounding environment [31]. However, cyanobacteria are less dependent on the host than other diazotrophs, such as rhizobia, due to the presence of specialized cells (i.e., heterocysts) and a cellular mechanism to reduce the oxygen concentration in the cytosol [32]. Nostoc species are heterocystic nitrogen-fixing cyanobacteria, producing motile filaments called hormogonia, and are considered the most common cyanobacteria in symbiotic associations [33,34]. The ability of diazotrophs cyanobacteria to fix nitrogen through various oxygen-sensitive enzymes, such as molybdenum nitrogenase (nifH), vanadium nitrogenase (vnfH), and iron-only nitrogenase (anfH), is a key point to fully understand the relationships between cyanobionts and their hosts [28].[2]
Multicellular organisms coevolved with a plethora of symbiotic microorganisms. These associations have a crucial effect on the physiology of both [35] and, in some cases, the host-associated microbiota can be considered as a meta-organism forming an intimate functional entity [36]. This means that there are coevolutive factors that led to the evolution of signals, receptors, and infochemicals among the organisms involved in symbiosis. Host–symbionts communication, based on this complex set of dose-dependent [37] and evolutionarily evolved [38] infochemicals, influences many physiological aspects of symbiosis; some examples are the microbiota composition, defensive mechanisms, development, morphology, and behavior (Figure 3) [39]. The main interactions occurring between cyanobacteria and host organisms are summarized in Table 1.[2]
Protists
Photosynthetic eukaryotes are the product of an endosymbiotic event in the Proterozoic oceans, more than 1.5 billion years ago [86,87]. For this reason, all eukaryotic phytoplankton can be considered an evolutive product of symbiotic interactions [87] and the chloroplast, as the remnant of an early symbiosis with cyanobacteria [86]. Nowadays, the associations among these unicellular microorganisms range from simple interactions among cells in close physical proximity, often termed “phycosphere” [88], to real ecto- and endosymbiosis. The study of these associations is often neglected, partially because symbiotic microalgae and their partners show an enigmatic life cycle. In most of these partnerships, it is unclear whether the relationships among partners are obligate or facultative [89]. The symbiotic associations between cyanobacteria and planktonic unicellular eukaryotes, both unicellular and filamentous, are widespread, in particular in low-nutrient basins [89]. It is assumed that cyanobacteria provide organic carbon through photosynthesis, taking advantage of the special environmental conditions offered by the host. In contrast, some single-celled algae are in symbiotic association with diazotrophic cyanobacteria, providing nitrogen-derived metabolites through N2 fixation [90]. This exchange is important for nitrogen acquisition in those environments where it represents a limiting factor, both in terrestrial and in aquatic systems, as well as in open oceans [91]. In fact, in marine environments, cyanobacteria are associated with single-celled organisms such as diatoms, dinoflagellates, radiolarians, and tintinnids [52,92]. The exchange of nitrogen between microalgae and cyanobacterial symbionts, although important, is probably flaked by other benefits such as the production of metabolites, vitamins, and trace elements [49,93]. In fact, available genomic sequences indicate bacteria, archaea, and marine cyanobacteria as potential producers of vitamins [94], molecules fundamental in many symbiotic relationships. Moreover, about half of the investigated microalgae have to face a lack of cobalamin, and other species require thiamine, B12, and/or biotin [95,96]; these needs may be satisfied, in many cases, by the presence of cyanobionts [97].[2]
The first case described of marine planktonic symbiosis was represented by the diatom diazotrophic associations (DDAs) among diatoms and filamentous cyanobacteria provided of heterocysts [98]. Although this kind of interaction is the most studied, little is known about the functional relationships of the symbiosis. Recent studies are mainly focused on the symbiotic relationships between the diazotroph cyanobacteria Richelia intracellularis and Calothrix rhizosoleniae with several diatom partners, especially belonging to the genera Rhizosolenia, Hemiaulus, Guinardia, and Chaetoceros [18,40]. The location of the symbionts varies from externally attached to partially or fully integrated into the host [41]. Indeed, it has been demonstrated through molecular approaches that morphology, cellular location, and abundances of symbiotic cyanobacteria differ depending on the host and that the symbiotic dependency and the location of the cyanobionts R. intracellularis and C. rhizosoleniae seems to be linked to their genomic evolution [99]. In this regard, it was demonstrated a clear relationship between the symbiosis of diatom–cyanobacteria symbiosis and the variation of season and latitude suggesting that diatoms belonging to the genus Rhizosolenia and Hemiaulus need a symbiont for high growth rates [40]. The reliance of the host seems closely related to the physical integration of symbionts: endosymbiotic relationships are mainly obligatory, while ecto-symbiosis associations tend to be more facultative and/or temporary [89]. Another interesting cyanobacteria–diatoms symbiosis involves the chain-forming diatom Climacodium frauenfeldianum, common in oligotrophic tropical and subtropical waters [100]. In this case, diatoms establish symbiotic relationships with a coccoid unicellular diazotroph cyanobacterial partner that is similar to Crocosphaera watsonii in morphology, pigmentation, and nucleotide sequence (16S rRNA and nifH gene) [41]. In addition, it has been demonstrated that nitrogen, fixed by cyanobionts is transferred to diatom cells [90]. Occasionally, C. watsonii has been reported as symbiotic diazotroph in other marine chain-forming planktonic diatoms, such as those belonging to the genera Streptotheca and Neostrepthotheca [42]. One of the most peculiar symbiosis is represented by the three-part partnership between the unicellular cyanobacterium Synechococcus sp., Leptocylindrus mediterraneus, a chain-forming centric diatom, and Solenicola setigera, an aplastidic colonial protozoa [43,44]. This peculiar association is cosmopolitan and occurs primarily in the open ocean and the eastern Arabian Sea; nevertheless, it remained poorly studied and exclusively investigated by means of microscopy techniques. Electron microscopy observations (SEM) reveal that in presence of S. setigera, the diatom can be apochlorotic (it lacks chloroplasts), thus offering refuge to the aplastidic protozoan, benefiting, and nourishing from the exudates it produces. It is assumed that the cyanobacterial partner, Synechoccus sp., supports the protozoan by supplying reduced nitrogen. It is also speculated that the absence of the cellular content of L. mediterraneus can be due to parasitism by S. setigera [44]. Recent studies reported a novel symbiotic relationship between an uncultivated N2-fixing cyanobacterium and a haptophyte host [45,46,47,48,49]. The host is represented by at least three distinctly different strains in the Braarudosphaera bigelowii group, a calcareous haptophyte belonging to the class of Prymnesiophyceae [101,102,103]. The cyanobiont, first identified in the subtropical Pacific Ocean through the analysis of nifH gene sequence, is UCYN-A or “Candidatus Atelocyanobacterium Thalassa,” formerly known as Group A. For many years, the lifestyle and ecology of this cyanobiont remained unknown, because cannot be visualized through fluorescence microscopy. Furthermore, the daytime maximum nifH gene expression of UCYN-A opposite with respect to unicellular diazotroph organisms [104,105]. The entire genome of the UCYN-A cells was sequenced, leading to the discovery of the symbiosis: the genome is unusually small (1.44 Mbp) and revealed unusual gene deletions, suggesting a symbiotic life history. Indeed, the genome completely lacks some metabolic pathways, oxygen-evolving photosystem II (PSII), RuBisCo for CO2 fixation, and tricarboxylic acid (TCA), revealing that the cyanobiont could be a host-dependent symbiont [47,48].[2]
Symbiotic relationships include interactions between cyanobacteria and nonphototrophic protists. Heterotrophic protists include nonphotosynthetic, photosynthetic and mixotrophic dinoflagellates, radiolarians, tintinnidis, silicoflagellates, and thecate amoebae [51,52,92,106,107]. In dinoflagellates, cyanobionts were observed using transmission electron microscopy with evidence of no visible cell degradation, the presence of storage bodies and cyanophycin granules, nitrogenase, and phycoerythrin (confirmed by antisera localization), confirming that these cyanobionts are living and active and not simple grazed prey [52,108,109]. In addition, these cyanobionts are often observed with coexisting bacteria, suggesting a potential tripartite symbiotic interaction [52,109]. A cyanobiont surrounding the outer sheath was observed in rare cases, suggesting an adaptation to avoid cell degradation in symbiosis [52]. Despite the presence of N2 fixing cyanobacteria, molecular analyses demonstrated the presence of a vast majority of phototrophic cyanobionts with high similarity to Synechococcus spp. and Prochlorococcus spp. [50,51]. The complex assemblage of cyanobacteria and N2 fixing proteobacteria suggests a puzzling chemical and physiological relationship among the components of symbiosis in dinoflagellates, with an exchange of biochemical substrates and infochemicals, and the consequent coevolution of mechanisms of recognition and intracellular management of the symbionts. In tintinnid, ciliates able to perform kleptoplastidy, epifluorescent observations of Codonella species demonstrated the presence of cyanobionts, with high similarities with Synechococcus, in the oral grove of the lorica and, in addition, the presence of two bacterial morphotypes [52]. In radiolarians (Spongodiscidae Dictyocoryne truncatum), the presence of cyanobionts has been demonstrated, initially identified as bacteria or brown algae [110,111]. In addition, several non-N2-fixing cyanobionts have been identified using autofluorescence, 16s rRna sequence, and cell morphology, resembling Synecococcus species [51,52]. In agreement with associations observed in dinoflagellates, mixed populations of cyanobacteria and bacteria are common in radiolarian species, although their inter-relationship is still unknown.[2]
Macroalgae and seagrasses
Mutual symbioses between plants and cyanobacteria have been demonstrated in macroalgae and seagrasses, as is the case of Acaryochloris marina and Lynbya sp., in which cyanobacteria contribute to the epiphytic microbiome of the red macroalgae Ahnfeltiopsis flabelliformis [53] and Acanthophora spicifera [54], respectively. Epiphytic relationships have been demonstrated as well with green and brown algae [112]. In Codium decorticatum, endosymbionts cyanobacteria belonging to genera Calothrix, Anabaena, and Phormidium, have been shown to fix nitrogen for their hosts [55,56]. Cyanobacteria are also common as seagrass epiphytes, for example, on Thalassia testudinum, where organic carbon is produced by cyanobacteria and other epiphyte symbiotic organisms rather than the plant itself [57,58]. In many cases, the presence of phosphates stimulates the cyanobionts growth on seagrasses and other epiphytes [113,114]. In oligotrophic environments, nitrogen-fixing cyanobacteria are advantaged against other seagrass algal epiphytes [115], and these cyanobacteria may contribute to the productivity of seagrass beds [116]. In addition, a certain level of host specificity can be determined in many plant–cyanobacteria symbioses [59], for example, among heterocystous cyanobacteria such as Calothrix and Anabaena, and the seagrass Cymodocea rotundata. A few cyanolichens live in marine littoral waters [92], and they play a role in the trophism of Antarctic environments, where nitrogen inputs from atmospheric deposition are low [117,118,119].[2]
Sponges
Marine sponges are among the oldest sessile metazoans, known to host dense microbial communities that can account for up to 40–50% of the total body weight [31]. These microbial communities are highly species-specific, and characterized by the presence of several bacterial phyla; cyanobacteria constitute one of the most important groups [120,121,122]. Sponges with cyanobionts symbionts can be classified as phototrophs when they are strictly depending on symbionts for nutrition or mixotrophs when they feed also by filter feeding [92]. These “cyanosponges” are morphologically divided into two categories—the phototrophs present a flattened shape, while the mixotrophs have a smaller surface area to volume ratio [29]. Cyanobacteria are located in three main compartments in sponges: free in the mesohyl, singly or as pairs in closed-cell vacuoles, or aggregated in large specialized “cyanocytes” [123]. Their abundance decreases away from the ectosome, while it is null in the endosome of the sponge host [124]. Cyanobacteria belonging to the genera Aphanocapsa, Synechocystis, Oscillatoria, and Phormidium are usually found in association with sponges and most species are located extracellularly, while others have been found as intracellular symbionts benefiting sponges through fixation of atmospheric nitrogen [92]. Indeed, some cyanobacteria located intracellularly within sponges showed to own nitrogenase activity [124]. Most of the sponges containing cyanobionts, however, are considered to be net primary producers [125]. Cyanobacteria in sponges can be transmitted vertically (directly to the progeny) or horizontally (acquired from the surrounding environment), depending on the sponge species [29]. For instance, the sponge Chondrilla australiensis has been discovered to host cyanobacteria in its developing eggs [126]. Caroppo et al., instead, isolated the cyanobacterium Halomicronema metazoicum from the Mediterranean sponge Petrosia ficiformis, which has been later found as a free organism and isolated from leaves of the seagrass Posidonia oceanica [119,127], highlighting that horizontal transmission of photosymbionts can occur in other sponge species [128]. Cyanobacteria associated with sponges are polyphyletic and mostly belonging to Synechoccoccus and Prochlorococcus genera [129]. Synechococcus spongiarum is one of the most abundant symbionts found in association with sponges worldwide [130,131]. In some cases, however, the relationship between symbionts and host sponges can be controversial. Some Synechococcus strains seem to be mostly “commensals”, whereas symbionts from the genus Oscillatoria are involved in mutualistic associations with sponges [3,132]. In the past, many researchers performed manipulative experiments to demonstrate the importance of cyanobacteria associations for the metabolism of the host [3,128,133]. A case study from Arillo et al. performed on Mediterranean sponges revealed that Chondrilla nucula, after six months in the absence of light, displayed metabolic collapse and thiol depletion [63]. This highlights that symbionts are involved in controlling the redox potential of the host cells transferring fixed carbon in the form of glycerol 3-phosphate and other organic phosphates. Instead, Petrosia ficiformis, which is known to live in association with the cyanobacterium Aphanocapsa feldmannii [62], showed the capability to perform heterotrophic metabolism when transplanted in dark conditions [63]. In some tropical environments, the carbon produced by cyanobionts can supply more than 50% of the energy requirements of the sponge holobiont [122]. Cyanobacteria, moreover, can contribute to the sponge pigmentation and production of secondary metabolites (e.g., defensive substances) [134], as in the case of the marine sponge Dysidea herbacea [64]. Thus, symbiotic associations could result in the production of useful compounds with biotechnological potential [134,135]. Meta-analysis studies on sponge–cyanobacterial associations revealed that several sponge classes could host cyanobacteria, although most of the knowledge in this field remains still unknown, and mostly hidden in metagenomics studies [136]. Sponge-associated cyanobacteria hide a reservoir of compounds with biological activity, highlighting an extraordinary metabolic potential to produce bioactive molecules for further biotechnological purposes [137].[2]
Cnidarians
It is widely accepted that reef environments rely on both internal cycling and nutrient conservation to face the lack of nutrients in tropical oligotrophic water [138]. A positive ratio in the nitrogen export/input between coral reefs and surrounding oceans has been observed [139,140]. Tropical Scleractinia are able to obtain nitrogen due to various mechanisms that include the endosymbiont Symbiodinium [141], the uptake of urea and ammonium from the surrounding environment [142], predation and ingestion of nitrogen-rich particles [143,144,145,146], or diazotrophs itself through heterotrophic feeding [147] and nitrogen fixation by symbiotic diazotrophic communities [4,7,68,69,73,148]. In addition to nitrogen fixation, coral-associated microbiota performs various metabolic functions in carbon, phosphorus, sulfur, and nitrogen cycles [74,149,150,151]; moreover, it plays a protective role for the holobiont [152,153,154], possessing inhibitory activities toward known coral pathogens [155]. These complex microbial communities that populate coral surface mucopolysaccharide layers show a vertical stratification of population resembling the structure of microbial mats, with a not-dissimilar flux of organic and inorganic nutrients [156]. It is reasonable to believe that microbiota from all the compartments, such as tissues and mucus, can contribute to the host fitness and interact with coral in different ways, ranging from the direct transfer of fixed nitrogen in excess to the ingestion and digestion of prokaryotes [20]. Diazotrophs, and in particular cyanobionts, are capable of nitrogen fixation and they can use glycerol, produced by zooxanthellae, for their metabolic needs [4,73]. The relationship between corals and cyanobacteria is yet to be fully explored and understood but some lines of evidence regarding Acropora millepora [69,70] suggest coevolution between corals and associate diazotrophs (cyanobionts). This relationship appears to be highly species-specific. In hermatypic corals, a three-species symbiosis can be observed, with diazotrophs in direct relation with Symbionidium symbiont. In Acropora hyacinthus and Acropora cytherea, cyanobacteria-like cells, characterized by irregular layered thylakoid membranes and with a remarkable similarity to the ones described by previous authors [4], were identified in strict association with Symbiodinium, within a single host cell, especially in gastrodermal tissues [67]. The high density of these cells closely associated with Symbiodinium suggests that the latter is the main user of the nitrogen compounds produced by the cyanobacterium-like cells. The presence of these cyanobacterium-like cells is more widespread than assumed in the past and this symbiosis was found in many geographic areas, for example, in the Caribbean region and the Great Barrier Reef [67].[2]
Microbial communities inhabiting the coral surface can greatly vary due to environmental conditions [147,157,158]. Diazotroph-derived nitrogen assimilation by corals varies on the basis of the autotrophic/heterotrophic status of the coral holobiont and with phosphate availability in seawater. Consequently, microbial communities increase when corals rely more on heterotrophy or when they live in phosphate-rich waters [147]. This suggests that diazotrophs can be acquired and their population managed according to the needs of corals [159]. This view was confirmed by the identification of a first group of organisms that form a species–specific, temporarily, and spatially stable core microbiota and a second group of prokaryotes that changes according to environmental conditions and in accordance with the host species and physiology state [160]. Experimental lines of evidence, using N2-labelled bacteria, demonstrated that diazotrophs are transferred horizontally and very early in the life cycle, and it is possible to identify nifH sequences, in larvae and in one-week-old juveniles [70], and in adult individuals [69] of the stony coral Acropora millepora. About coral tissues, the distribution of microbiota, and cyanobacteria as well, is not the same in all the tissue districts. Species that live in the mucus resemble the species variety and abundance that can be found in the surrounding water. On the contrary, the microbiota of internal tissues including also calcium carbonate skeletons is made, at least partially, of species that cannot be easily found free in the environment [68,69]. This plasticity might as well characterize cyanobacteria hosted in cnidarians, although such multiple relationships are still scarcely investigated.[2]
Synechococcus and Prochlorococcus cyanobacteria have been identified in association with Montastraea cavernosa [4], through molecular approaches and genes belonging to filamentous cyanobacteria [6]. Filamentous and unicellular diazotrophic cyanobacteria belonging to the orders Chroococcales, Nostocales, Oscillatoriales, and Proclorales were found, using pyrosequencing approach, as associated organisms to the shallow water coral Porites astreoides [6] and Isopora palifera [71]. On the contrary, in Montipora flabellate, Montipora capitate [7], Acropora millepora [69,70], Acropora muricate, and Pocillopora damicornis [69], cyanobacteria are present in various tissues and in the skeleton, but their contribution in terms of nitrogen fixation is minimal [5]. In Montastraea cavernosa, Montastraea franksi, and in species of the genus Diploria and Porites, cyanobacterial sequences belonging to various genera (e.g., Anabaena, Synechoccus, Spirulina, Trichodesmium, Lyngbya, and Phormidium) have been found in coral tissues by PCR amplification [4,73,74,75,161]. In Montastraea cavernosa, the orange fluorescence protein, peaking at 580 nm, was attributed to phycoerythrin, a cyanobacterial photopigment produced by a cyanobacterium living in the host epithelial cells [4]. The different colors, especially of fluorescent proteins in corals, suggest specific biological functions for these compounds. Moreover, it is not clear if they act as photoprotective compounds, antenna pigments, or if they photoconvert part of the light spectrum to help zooxanthellae photosynthesis. These results are contested by some authors who excluded the role of phycoerythrin as a pigment compound in corals [5]. In order to determine the presence and the activity of cyanobacteria in corals, the following aspect should be considered: nonquantitative approaches cannot assure accurate values of abundance; moreover, the presence of nifH gene is not necessarily linked to the fixation and the transfer of nitrogen performed by diazotrophs. H [20]. Endolithic cyanobacteria have been found in Porites cylindrica and Montipora monasteriata, but their role in the relationship with host corals is unknown [162]. In contrast, in other cnidarians, it has been demonstrated that endolithic cyanobacteria establish symbiotic relationships with coral hosts: this is the case of Plectonema terebrans, a cyanobacterium belonging to the order Oscillatoriales [72]. Cold-water corals are ecosystem engineers providing a habitat for thousands of different species. Their trophism is related to the low energy, partially degraded, organic matter that derives from the photic zone of oceans [163]. To face the lack of nutrients, cold-water corals evolved, on one hand, from an opportunistic feeding strategy [164,165], and on the other hand, from a symbiosis with various diazotrophs, including cyanobacteria [166,167,168]. Plectonema terebrans filaments, visible as pinkish to violet staining, are able to colonize the entire skeleton of the cold-water corals Desmophyllum dianthus and Caryophyllia huinayensis; however, their density is higher at the skeleton portion covered with polyp tissue [72]. The close contact between coral tissues and cyanobacteria obliges the endoliths to exchange nutrients with the surrounding water through the polyp itself. This close relationship is advantageous for the cyanobacterium because the coral nematocysts protect it from the grazers [169], and it is mutualistic because such a close relationship inevitably includes exchanges of metabolites between organisms [170]. These metabolites produce benefits for the host and play a trophic and/or protective role in the symbiotic mutualistic relationship. Middelburg et al. suggested that in cold-water corals, a complete nitrogen cycle occurs similar to that inferred for tropical reefs, ranging from ammonium production and assimilation to nitrification, nitrogen fixation, and denitrification [166].[2]
The effects of environmental changes on the nitrogen fixation rates are still poorly explored, especially if specifically related to the symbiotic diazotrophs and to cyanobacteria. Ocean acidification enhances nitrogen fixation in planktonic cyanobacteria, as in the case of Crocosphaera watsoni, due to enhancement of photosynthetic carbon fixation [171]. It is interesting to underline that in the planktonic diazotroph cyanobacterium Trichodesmium sp., which forms symbiotic association with diatoms [172], the nitrogen fixation is enhanced under elevated CO2 conditions [173], but it is strongly reduced if there is an iron limitation [174]. On the contrary, Seriatopora hystrix diazotrophs are sensible to ocean acidification, with a decline of the nitrogen fixation rate at high CO2 concentration, leading to consequences on coral calcification and potential starvation for both the coral and the Symbiodinium spp. [175]. In addition, environmental changes can increase in coral symbionts, the abundance of microbial genes involved in virulence, stress resistance, sulfur and nitrogen metabolisms, and production of secondary metabolites. These changes that affect the physiology of symbionts can also affect the composition of the coral-associated microbiota [74], with the substitution of a healthy-associated coral community (e.g., cyanobacteria, Proteobacteria), playing a key role in mediating holobiont health and survival upon disturbance [176], with a community related to coral diseases (e.g., Bacteriodetes, Fusobacteria, and Fungi).[2]
Ascidians and other tunicates
Tunicates are considered rich in biologically active secondary metabolites [177,178,179,180], but it is unclear if these bioactive compounds were produced by tunicates themselves or by associated microorganisms [181,182], although strong direct and indirect lines of evidence show that defensive compounds and other secondary metabolites are produced by various symbiotic prokaryotes and not by the tunicates themselves. Among tunicate symbionts, cyanobacteria have been found in symbiotic relationships with various tunicates, ranging from tropical to temperate environments. In fact, obligate associations with cyanobacteria of Prochloron and Synechocystis genus have been found in some species of ascidians belonging to the genera Didemnum, Lissoclinum, Diplosoma, and Trididemnum [77], with cyanobacterial cells distributed in the cavities and/or tunic [78]. These cyanobionts have been demonstrated to be part of the core microbiome, in which species and populations do not reserve the water–column ones and microbiome–host relationship is species specific and not correlated to the geographical location [9]. In colonial ascidians, such as Botryllus schlosseri and Botrylloides leachii, an abundant population of Synechococcus-related cyanobacteria have been identified [79], while in the Mediterranean ascidian Didemnum fulgens, a coral-associated cyanobacterium has been observed in its tissues [183]. In some cases, the cyanobiont completely or partially lacks the nitrogen-fixation pathway. This is the case of Prochloron didemni, in symbiosis with the tunicate Lissoclinum patella, which is probably involved in carbon fixation and in the ammonia incorporation and not in the nitrogen fixation [80,81]. In fact, in contrast with the presence of genes for the nitrate reduction pathway and all primary metabolic genes required for free-living, Prochloron seems to lack the capability to fix nitrogen and to live outside the host [80]. Prochloron sp. also protects the host versus active forms of oxygen, which can be formed during photosynthesis processes. The cyanobacterium produces a cyanide-sensitive superoxide dismutase, a Cu-Zn metalloprotein, that has been demonstrated to prevent the toxicity of superoxide radicals, hydrogen peroxide, and hydroxyl radicals in the host ascidians [82]. In Lissoclinum patella, other cyanobacteria were abundant in various tissues and one of these is Acaryochloris marina, a chlorophyll d-rich cyanobacterium, able to sustain oxygenic photosynthesis under near-infrared radiation that propagates through Prochloron cells and ascidian tissue [83]. The Caribbean tunicate Trididemnum solidum produces a peculiar biologically active molecule, the acyl-tunichlorine (Figure 2) [84,85], that contains both nickels accumulated by the tunicate and pheophytin, which is produced by organisms with photosynthetic machinery and suggests a dual origin of this compound. In fact, this tunicate hosts the cyanobacterium Synechocystis trididemni, which contributes to the production of acyl-tunichlorine synthesizing the pheophytin through an intermediate molecule, the pyropheophorbide [84,85]. In addition, behavioral tests demonstrated the presence of deterring compounds in ascidian larvae able to distaste predatory fishes. These compounds have been identified to be didemnin B (Figure 2) and nordidemnin [65]. Didemnin B was found in various tunicates, and it is similar to a bioactive molecule produced by other cyanobacteria, enforcing the idea that the predation-deterring compounds can be produced by cyanobionts [184], although the possibility of a horizontal gene transfer cannot be totally rejected [185,186]. The tunicate–cyanobacteria symbiosis is evidenced by the presence, in the host tunicate, of a cellulose synthase gene, similar to the one found in cyanobacteria, which probably derives from horizontal transfer between the two organisms [187,188] and that may have a role in the tunicates evolutive radiation and in the development of adult and larvae body plans [188,189,190]. The presence of a rich and bio-diversified microbiome makes tunicates promising models for various purposes and important for drug discovery [10,191].[2]
References
- ISSN 1932-6203..
{{cite journal}}: CS1 maint: unflagged free DOI (link) Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License - ^ ISSN 1660-3397..
{{cite journal}}: CS1 maint: unflagged free DOI (link) Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License
Sea surface microlayer processes
As with other marine ecosystems, life in the SML is dominated by microorganisms, which are collectively referred to as the “neuston,” whereas “plankton” describes the microscopic organisms that inhabit the underlying water column. So far, most recent studies have focused on the diversity and abundance of bacterioneuston assemblages, principally through the application of molecular ecology tools (for a review see Cunliffe et al., 2011). The consensus view is that bacterioneuston assemblages form by recruitment from the underlying bacterioplankton; however, the species composition and activity of bacterioneuston assemblages can be very different compared to those in the underlying water column (Agogue et al., 2005; Franklin et al., 2005; Obernosterer et al., 2008; Stolle et al., 2011; Taylor et al., 2014). What exactly controls bacterioneuston species diversity is at present not fully understood, but evidence from both field observations (Cunliffe et al., 2009a) and large-scale mesocosm experiments (Cunliffe et al., 2009c) suggests that the development of bacterioneuston assemblages is ecologically regulated and not random (Cunliffe et al., 2011). Possible controls of bacterioneuston diversity and activity include the prevailing meteorological conditions, incident UV light, organic matter availability and aerosol deposition (Carlucci et al., 1985; Agogue et al., 2005; Stolle et al., 2010, 2011; Nakajima et al., 2013; Astrahan et al., 2016). It is, however, not clear whether solar and UV irradiance promote or inhibit neuston activity (Bailey et al., 1983; Carlucci et al., 1985; Santos et al., 2012).[1]
The bacterioneuston has been shown to directly influence air-sea gas exchange by consuming and producing trace gases (e.g., CO, H2, CH4, N2O) (Conrad and Seiler, 1988; Frost, 1999; Upstill-Goddard et al., 2003; Nakajima et al., 2013). The addition of methanotrophs in a laboratory gas exchange tank led to an enhancement of the apparent transfer velocity of CH4 by 12 ± 10% (Upstill-Goddard et al., 2003). Later work reported bacterial growth efficiency in the SML and underlying water to be similar, but also showed higher respiration in the SML that pointed to some potential control of the air-sea exchange of CO2 and O2 by the bacterioneuston (Reinthaler et al., 2008). Through the application of functional gene probes to a range of SML environments, the metabolic potential for trace gas cycling in the SML has since been established as being widespread (Cunliffe et al., 2008, 2011). It can therefore be concluded that for some trace gases such as CH4 and CO2, the bacterioneuston is intimately involved in their cycling and that it may potentially act as either a small gas source or sink in response to the prevailing microbial and biogeochemical conditions. The bacterioneuston may also modulate the enrichment of surfactants in the SML and thus indirectly influence air-sea gas exchange, as indicated by a recent study relating SML surfactant enrichment and the occurrence of specific bacterial taxa (Kurata et al., 2016).[1]
The second major biological component of SML ecosystems is phytoneuston, historically studied using microscopes (Hardy and Valett, 1981; Hardy, 1982), and more recently through high-throughput sequencing (Taylor and Cunliffe, 2014). High-throughput sequencing of phytoneuston assemblages in the coastal SML of the English Channel showed that biodiversity at the interface was very different to that in the underlying water (Taylor and Cunliffe, 2014). Two major algal groups, the chrysophytes and the diatoms, were significantly enriched in the neuston, with diatom diversity in particular being distinctly different to that of planktonic diatoms. While the functional impacts of the differences between phytoneuston and phytoplankton abundance and diversity are not yet fully understood, it is believed that specific processes such as the maintenance of air-sea CO2 disequilibria could be controlled, at least in part, by the phytoneuston (Calleja et al., 2005).[1]
Other components of SML ecosystems such as heterotrophic protists and zooneuston have also been studied, but to a lesser extent. Looking forward, the application of contemporary tools, such as high-throughput sequencing, hold great promise in revealing new insights into the biology of the SML. The recent English Channel study discussed above also showed that the diversity of neustonic fungi (myconeuston) is also distinctly different from mycoplankton diversity (Taylor and Cunliffe, 2014). Relative to other marine microbial groups, the marine fungi remain poorly understood. A recent study of the coastal mycoplankton implicated marine fungi in a range of ecosystem functions, including nutrient recycling and parasitism (Taylor and Cunliffe, 2016). Exactly what functional roles myconeuston may play at the ocean-atmosphere interface still remain to be revealed.[1]
Marine aerosols that are formed from the SML via bubble bursting are a vector for the transfer of microbial life from the lower water column and SML into the atmosphere. Measurements of bacteria and viruses in sea spray aerosols (SSA) show large enrichments relative to both the SML (~5) and subsurface waters (~10) (Aller et al., 2005). A key factor in this process appears to be the embedding of microorganisms within the organic gel matrix that forms in the SML (discussed further below) (Aller et al., 2005). The global-scale implications for ocean-atmosphere exchange of microorganisms are profound because this process may have a major impact on the long-distance dispersal of marine microbial life. For example, phytoplankton viruses that are transported into the atmosphere from the SML can remain highly infective under prevailing meteorological conditions and can be spread across hundreds of kilometers to subsequently infect distal phytoplankton (Sharoni et al., 2015).[1]
Organisms in the SML interact with the surface accumulation of organic matter produced by biological processes in the underlying water column (Galgani et al., 2014). Some dissolved compounds have surface active (surfactant) properties or adsorb onto floating compounds and selectively become enriched in the SML (Hunter and Liss, 1977). The accumulation of surfactants in the SML was found to be related to the occurrence of certain bacterioneuston taxa, indicating microbial contribution to surfactant production and decomposition (Kurata et al., 2016). In addition to soluble surfactants, the SML also accumulates a variety of colloidal and particulate organic matter that may serve as substrates for the bacterioneuston. Sieburth (1983) proposed the SML to be a complex hydrated “gel” of macromolecules and colloidal material.[1]
The “gel” material has now been identified and studied in greater detail. Included in the gelatinous matrix are transparent exopolymer particles (TEP), i.e., polysaccharide-rich microgels that are produced primarily by the abiotic coagulation of phytoplankton exudates (Passow, 2002), and Coomassie stainable particles (CSP) containing proteinaceous polymers released during cell lysis and decomposition (Long and Azam, 1996). The aggregation of TEP precursors in the SML is enhanced due to the constant compression and dilation of the air-sea interface (Wurl et al., 2011), which may explain, at least in part, the gelatinous nature of the SML. Gel particles may serve as grazing protection for the phytoneuston and bacterioneuston, as has been shown for their planktonic counterparts (Passow and Alldredge, 1999; Dutz et al., 2005). In addition, TEP have been suggested to protect neuston from high irradiance (Ortega-Retuerta et al., 2009), possibly helping the neuston withstand periods of high irradiation of the SML.[1]
Surface microlayer

The surface microlayer (SML) is an ecotone, which constitutes an interface between the hydrosphere and the atmosphere. It is a thin layer of maximum 1000 µm in thickness [1]. The SML is unique due to its physical, chemical and biological properties, which differ from those of subsurface water (SUB) [1,2]. The SML is capable of accumulating microorganisms and chemical substances at a rate as high as 100-fold greater than that observed in the SUB [3]. The SML is the zone of matter and energy exchange between the hydrosphere and the atmosphere, and, as such, it is affected by global climate changes [4].[3]
The accumulation of chemical substances in the SML may vary, as it is dependent on many factors such as the chemical composition of water, salinity, the nutrient status and the presence of neustonic organisms (organisms that live upon the upper surface of waters or beneath its surface film, the SML) [5,6]. The SML may also accumulate toxic substances at greater amounts than observed in the pelagic zone [7]. At the same time, it is the zone that accumulates nutrients [5,8]. In turn, such substances as calcium, magnesium, potassium or sodium, being macroelements found at high concentrations in the pelagic zone, are usually not accumulated in the SML [9]. The SML is inhabited by neustonic autotrophic and heterotrophic microorganisms, which exhibit both enzymatic and high respiratory activity [5,10,11,12]. The SML structure may be disturbed as a result of physical non-equilibrium processes such as temperature fluxes, irradiance, wind and wave action that influence its biogeochemical properties. However, it is well fit for a rapid self-reconstruction of its original structure [3]. Physical forces such as adhesion, cohesion, surface tension, vortex motion and the Langmuir circulations, associated with its unique chemical and biological structure, form the specific properties of SML, including its stability [2].[3]
Chemical contaminants are accumulated in the SML because of physical, chemical and biological processes. These processes include, e.g., hydrosphere–atmosphere exchange, convection, upwelling of underlying waters, transport by bubbles, atmospheric deposition, simple diffusion, buoyant particles, turbulent mixing, scavenging and chelation of inorganic components by organic matter [3]. Important sources of contaminants in urban objects are connected with urban pollutants supplied with wet and dry atmospheric deposition [13,14].[3]
The taxonomic composition of phytoplankton is dependent on the chemical composition of the aquatic environment, which it colonizes [5,15]. Consequently, communities of the phytoneuston and phytoplankton vary in the SML and the SUB in terms of their taxonomic composition and population size [16].[3]
Ocean surface ecosystem
Above and below
Air-sea interface
Food webs

Organisms that live at the surface are a nexus for food webs both above and below (Fig 3). From the air, seabirds prey on neuston, including fulmars, storm petrels, and sooty shearwaters (see review in [32]). For the Pacific ocean Laysan albatross, nearly 30% of their diet is neuston, including Velella, Janthina, Halobates, and the eggs and larvae of flying fish [10]. Even ducks [33] and sea-going bats [34] prey on floating neuston when they drift close to shore. Below the surface, diverse sea turtles eat neuston (see review in [32]), including olive ridley (Lepidochelys olivacea), which prey upon Janthina [9,35], green turtles (Chelonia mydas), which prey upon Porpita [36], and loggerhead turtles (Caretta caretta), which prey upon Velella and Janthina [9]. Neuston are among the most important prey for central North Pacific loggerhead sea turtles [9]. Fish like coho salmon Oncorhynchus kisutch and spiny dogfish Squalus acanthias prey on Velella (see review in [32]), and animals of the deep-scattering layer also prey upon neuston [37]. Diverse larval fish from a wide variety of ecologically and economically important species live as or prey on neuston [11] (Table 1).[5]
Neuston themselves reach into the waters below, capturing non-neustonic prey and further linking deeper waters to this thin surface layer. Velella feed on a variety of foods, including fish eggs and larvae [69], while Porpita and
Many species of the neuston also prey on one another, creating an interconnected food web stretching into the broader world around it. Janthina and Glaucus prey on Physalia, Velella, and Porpita. Janthina have also been observed trying to eat each other, suggesting they have the capacity to be cannibalistic [65]. The only true open ocean insect, the neustonic Halobates, preys upon other neuston by sucking nutrients from organisms with piercing mouthparts [72].[5]
Ecoregions
The ocean’s surface possesses diverse floating ecosystems within different regions. The only well-known neustonic ecoregion, the Sargasso Sea, covers an area in the western North Atlantic where the neustonic Sargassum concentrates. Multiple endemic species live in the Sargasso Sea, many of them adapted to shelter among the neustonic seaweed [14,15]. The Sargasso Sea contributes to a variety of ecosystem goods and services, and its valuation ranges from over US$200 million for fisheries services to US$2.7 billion for all services [16]. The distribution of surface life in the Sargasso Sea changes by season [17,18] and may be subject to annual and decadal trends [19,20]. These trends can impact both the ecology and economy of the region, as well as stakeholders further afield that rely on species that shelter in the Sargassum.[5]
Beyond the Sargasso Sea, the most detailed survey of Pacific neuston occurred in the 1950s. Scientists in the USSR crisscrossed the Pacific, collecting nearly 500 samples from 50°N to over 40°S [12]. In this massive survey, 7 distinct ecoregions were discovered, with different species of neuston showing different ranges that likely reflect their ability to move with wind, thermal optima, seasonality, and life cycles [12].[5]
A linear survey from Fiji to the Bay of Biscay also found considerable geographic variation. The tropical seas of the Indian Ocean were dominated by neustonic species including Halobates, Physalia, Velella, and Porpita [21]. In contrast, the eastern North Atlantic was dominated by small quick-moving crustaceans that made up over 90% of neustonic organisms [21,22]. This suggests these regions have distinct neustonic communities.[5]
Ecological variation across regions also includes how the surface changes through time. On short time scales, the surface habitat is part of the diel vertical migration of marine life from the deep sea: the largest migration on Earth, which happens twice each day [23]. Because of this migration, significant differences in surface life occur between day and night at basin-wide scales. Ostracods, mysids, isopods, heteropods, various crustacean and bryozoan larvae, are all more abundant at the surface at night [21,22]. In contrast, some surface-associated species, such as Sapphirina copepods, which use complex visual cues for mating, migrate to the surface only during the day [24]. These migratory species add to the diversity at the ocean’s surface. On larger time scales, neustonic Sargassum abundance changes seasonally [19,25,26], and some neuston, such as Velella, strand more often in certain seasons than others [27], possibly due to seasonal variation in distribution.[5]
Differences in neuston across space and time may be due to real population and species boundaries. For example, while some species, such as the nudibranch Glaucus atlanticus, are globally distributed, closely relatives Glaucus bennettae and Glaucus mcfarlanei have thus far been identified only in the North Pacific subtropical gyre system [28], and represent cryptic species. The sea skater Halboates shows remarkable population- and species-level isolation both across oceans and ocean basins [29,30], while neustonic Sargassum represent a genetic and morphotype species complex with diverse and distinct distribution patterns [31]. It is clear that neuston are not uniformly distributed, and there is evidence for both species and population isolation as well as sympatric speciation. However, for the majority of neustonic species, no genetic or population data exist. Are individuals of the “same species” half a planet away part of an interconnected global population, or isolated and distinct enough to be considered different species with unique adaptations to the conditions in their region of the world?[5]
Poorly studied neuston ecoregions should be considered in the context of the Sargasso Sea: We know this comparatively well-studied region is critical for both the ecology and economy of the North Atlantic, its services valued in the billions. What ecological and economic services are neuston ecosystems providing in other ocean regions?[5]
Threats
The ocean surface is a concentrating front for floating pollutants from plastic to petroleum. Metals and toxicants concentrate on the ocean’s surface, particularly
One large threat to both coastal and open-ocean surface organisms comes from oil. An estimated 741 kilotonnes of oil is released into the ocean each year from both natural and human sources,[8] with unknown effects on surface ecosystems. Because hydrophobic molecules concentrate at the ocean’s surface,[9][10] neustonic species will face orders of magnitude higher oil exposure than animals even a meter below the surface. Additionally, neuston species may also be vulnerable to dispersants used in breaking down oil spills, as is the case with jellyfish,[11] which die at significantly higher rates in the presence of dispersants, and Sargassum, which sinks in the presence of dispersants.[12] However, as of 2021 studies on diverse neustonic species in the presence of oil or dispersants have not been conducted.[5]
Floating plastic is another widespread petroleum product on the ocean’s surface.[13][14] There are an estimated 14.9 to 51.2 trillion pieces of plastic on the ocean’s surface,[15] representing upwards of 250,000 tons, largely concentrated in oceanic subtropical gyres (known colloquially as "Garbage Patches", which includes the Sargasso Sea).[16] These plastics are consumed by surface-hunting species like the Laysan albatrosses of Midway Atoll, which feed nearly 5 tons of ocean plastic to their chicks each year.[17][18] Such high plastic consumption makes sense only in light of these birds’ predation on neuston [10]. Larval neustonic fish and rafting barnacles have been found with plastic in their gut [11,97], though the impact of this plastic on these organisms, or the animals that feed on them, is not known. Some neustonic species, such as Halobates, may benefit from plastic, which provides a hard surface for laying eggs [98]. Larval fish may also shelter around plastic debris [73].[5]
With this complexity in mind, we must proceed cautiously when attempting to restore or conserve the ocean’s surface. For example, multiple organizations pledge to remove plastic from the ocean using unmanned collection devices inspired by pool skimmers or technology used to catch algae and jellyfish [99]. It should be no surprise then that one organization trapped hundreds of neustonic animals in their prototype, visible in their press release photo [100].[5]
Of all the human impacts on the ocean’s surface, climate change will have the farthest reach, and it is unclear what impact it will have on neuston. The ocean’s surface is directly exposed to the atmosphere, and changes in temperature will be felt first at the surface. This region is also uniquely exposed to atmospheric carbon dioxide and the ravages of storms, which are predicted to increase in intensity and frequency under climate change [101].[5]
Overall, threats to the neuston are poorly understood, and for every likely threat listed here, there are no doubt many that are largely unknown (e.g., ballast water, localized pollution, deep-sea mining impacts on pelagic life-cycle stages, geoengineering, etc.).[5]
Gallery
not a jellyfish but a colony of smaller organisms
-
Portuguese man-o-war Physalia sp.
-
By-the-wind sailor Velella sp.
-
Blue button Porpita sp.
-
Flying fish from the family Exocoetidae
-
Buoy barnacle Dosima fascicularis
-
Blue sea dragons Glaucus sp.
-
Paper nautilus Aurgonaut sp.
-
Sargassum sp. seaweed
-
Hippolytidae shrimp
-
Marine snail Recluzia sp.
-
Violet snail Janthina sp.
-
Floating anemone Actinecta sp.
References
- ^ a b c d e f g Cite error: The named reference
Engel2017was invoked but never defined (see the help page). - doi:10.5194/acp-2020-416)
{{citation}}: CS1 maint: unflagged free DOI (link - ^ doi:10.3390/w12071904..
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help)CS1 maint: unflagged free DOI (link) Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License - doi:10.5194/acp-2020-416)
{{citation}}: CS1 maint: unflagged free DOI (link - ^ a b c d e f g h i j k l m n o p q Cite error: The named reference
Helm2021was invoked but never defined (see the help page). - .
- ^ PMID 15172807.
- PMID 25057607.
- ^ Cite error: The named reference
Hardy1982was invoked but never defined (see the help page). - .
- PMID 26547023.
- PMID 24086378.
- S2CID 52086328.
- S2CID 206784554.
- S2CID 206784554.
- PMID 25494041.
- PMID 22575495.
- ^ Klavitter, J. (2005) "Calculation of the amount of plastic 'land filled' each year by albatross at Midway Atoll NWR". US Fish and Wildlife Service, Honolulu.
Microbiota
Microbiome dark matter
Research, for example on the human microbiome, has mainly been restricted to the identification of most abundant microbiota associated with health or disease. Their abundance may reflect their capacity to exploit their niche, however, metabolic functions exerted by low-abundant microrganisms can impact the dysbiotic signature of local microbial habitats.[1]
Advances in high-throughput sequencing approaches have revolutionised microbiology and enabled the characterization of the complex ecological contents of microbial communities, however, our understanding of the mechanisms impacting host-microbial homeostasis remains limited (Hajishengallis et al., 2012). Changes to the human gut microbial composition, for example, can influence host health and diseases, and may affect the microbiota at other body sites (Banerjee et al., 2018). A concept of pathogenicity influenced by both microorganisms and the host has been proposed in the damage-response framework (Casadevall and Pirofski, 2003).[1]
Research on the human microbiome has mainly been restricted to comparisons of the most abundant organisms and the identification of a “core” microbiota associated with health or disease. Indeed, the core microbiome may reflect their capacity to exploit their niche, being favoured by nutrients, O2 concentrations, etc. to allow surface colonisation. However, opportunistic pathogens may contribute to the compositional and or functional shift towards dysbiosis and could be among the minority taxa. Key species could therefore easily be overlooked in next generation sequencing (NGS) analyses (Turnbaugh et al., 2007; Zerón, 2014).[1]
Furthermore, studies using a 16S rRNA metagenomic approach are limited to the identification of bacteria and archaeae (arguably accurately to the genus level), leaving the view of the richness and diversity of the whole microbiome incomplete and underestimated (Brooks et al., 2015). This is certainly true for Methanobrevibacter smithii, a member of the Archaea domain in a relatively minor constituent of the gut microbiome that contributes to bacterial metabolism in ways that promote host dysbiosis (Hajishengallis et al., 2012). This species and its methanogenic relatives, though in low abundance, have been demonstrated to be capable of providing conditions for the growth of pathogenic bacteria in periodontal sites, driving to periodontitis (Lepp et al., 2004). The composition of the microbial communities can be misinterpreted regarding the presence of virus, archaea, and fungi, making it a challenge to gain a holistic view.[1]
Subsequently, low-abundant microrganisms could be considered the “dark matter” of the human microbiome. Recent studies (Hajishengallis and Lamont, 2016; Wang et al., 2017; Banerjee et al., 2018; Stobernack, 2019; Berg et al., 2020; Xiao et al., 2020) are paying more attention to these organisms, and increasingly taking into account the “keystone species” concept, corresponding to organisms which effect on the community is disproportionately large compared to their relative abundance (Power et al., 1996). A similar concept in macroecology suggests species in low abundance have a major role in their respective community (Hajishengallis et al., 2012). Abundance is the factor differentiating keystone microorganisms from those that are dominant. A dominant species might affect the environment exclusively by its sheer abundance, while a keystone microorganism may influence metabolic functions of the microbiome, despite its low abundance. Examples of keystone pathogens are: Porphyromonas gingivalis associated with periodontitis (Holt and Ebersole, 2005; Perez-Chaparro et al., 2014; Burmistrz et al., 2015; Camelo-Castillo et al., 2015; Ai et al., 2017; Stobernack, 2019), Klebsiella pneumonia, Proteus mirabilis (Garrett et al., 2010), and Citrobacter rodentium (Bry et al., 2006) associated with intestinal inflammatory diseases; and Fusobacterium nucleatum (Kostic et al., 2013; Rubinstein et al., 2013) associated with colon cancer (Banerjee et al., 2018). Furthermore, studies investigating Bacteroides fragilis, a pro-oncogenic bacterium, have found it to be a minor constituent of the colon microbiota in terms of relative abundance. Its unique virulence characteristics, such as secretion of a zinc-dependent metalloprotease toxin, alter colonic epithelial cells and mucosal immune function to promote oncogenic mucosal events, in which in addition to the intraluminal environment, enhance the oncogenic process. This gave rise to the concept of “alpha-bugs”, due to its ability to be directly pro-oncogenic but also to be capable of remodeling the entire healthy microbiota (Sears and Pardoll, 2011; Hajishengallis et al., 2012). Thus, the identification of low-abundant organisms within a microbial population associated with disease could be crucial. Unless we have a more “complete” view of the microbiota, including an accurate detection of low-abundant species, our understanding of the microbiology remains limited, as well as our strategy to improve therapy designs/interventions in diseases with polymicrobial cause.[1]
Studies of the minority microrganisms may reveal unique signatures, which could lead to diseases. Hence, a much deeper characterization of their presence in the microbiome in which they are involved is desirable. This scoping review aims to map the literature regarding the management of low-abundant organisms in studies investigating human samples. We aimed to determine: 1) How researchers classify organisms as low-abundant; 2) How they handled and processed NGS data of low-abundant organisms bioinformatically and 3) The distibution of low-abundant microorganisms among various body sites.[1]
Microswimmers
HEADERS...
- Overview
- Natural microswimmers
- Synthetic microswimmers
- Hybrid microswimmers
- Hydrodynamics
- Applications
SEE...
- Bioinspired reorientation strategies for application in micro/nanorobotic control[2]
- Microfluidics for Microswimmers[3]
- Microfluidic techniques for separation of bacterial cells via taxis[4]
- Opto-thermoelectric microswimmers[5]
"An artificial microswimmer is a cutting-edge technology with engineering and medical applications. A natural microswimmer, such as bacteria and sperm cells, also play important roles in wide varieties of engineering, medical and biological phenomena. Due to the small size of the microswimmer, the inertial effect of the surrounding flow field may be negligible. In such a case, reciprocal body deformation cannot induce migration of a swimmer, which is known as the scallop theorem. To overcome the implications of the scallop theorem, the microswimmer needs to undergo a nonreciprocal body deformation to achieve migration. The swimming strategy is thus completely different from macro-scale swimmers, which should be clarified much further." - Special Issue "Microswimmer"
- smart microswimmers
- Roads to Smart Artificial Microswimmers[6]
Artificial microswimmers offer exciting opportunities for biomedical applications. The goal of these synthetics is to swim like natural micro-organisms through biological environments and perform complex tasks such as drug delivery and microsurgery. Extensive efforts in the past several decades have focused on generating propulsion at the microscopic scale, which has engendered a variety of artificial microswimmers based on different physical and physicochemical mechanisms. Yet, these major advancements represent only the initial steps toward successful biomedical applications of microswimmers in realistic scenarios.[6]
A next step is to design “smart” microswimmers that can adapt their locomotion behaviors in response to environmental factors. Herein, recent progress in the development of microswimmers with intelligent behaviors is surveyed in three major areas: 1) adaptive locomotion across different media, 2) tactic behaviors in response to environment stimuli, and 3) multifunctional swimmers that can perform complex tasks. The emerging technologies and novel approaches used in developing these “smart” microswimmers, which enable them to display behaviors similar to biological cells, are discussed.[6]
- control over directionality
- Topographical pathways guide chemical microswimmers[7]

Achieving control over the directionality of active colloids is essential for their use in practical applications such as cargo carriers in microfluidic devices.[7]
Catalytically active micrometre-sized objects can self-propel by various mechanisms including bubble ejection, diffusio- and electrophoresis, when parts of their surface catalyse a chemical reaction in a surrounding liquid. In future, such chemically active micromotors may serve as autonomous carriers working within microfluidic devices to fulfill complex tasks1,2,3. However, to achieve this goal, it is essential to gain robust control over the directionality of particle motion. Although it has been more than a decade since motile chemically active colloids were first reported4,5,6,7, this remains a challenging issue, in particular for the case of spherical particles.[7]
Two main methods of guidance have been so far employed with varying degrees of success. The first one uses controlled spatial gradients of ‘fuel’ concentration. This approach suffers, however, from severe difficulties in creating and maintaining chemical gradients, and the spatial precision of guidance remains rather poor8,9,10,11,12. The second approach relies on the use of external magnetic fields in combination with particles with suitably designed magnetic coatings or inclusions5,13. This proved to be a very precise guidance mechanism, which could be employed straightforwardly for the case of tubular particles14, but difficult to extend to the case of spherical colloids, where it requires sophisticated engineering of multilayer magnetic coatings7,15,16,17. In addition, individualized guidance of specific particles is difficult to achieve without complicated external apparatus and feedback loops18. The advantages of autonomous operation are thereby significantly hindered.[7]
- Translational prospects of untethered medical microrobots[8]
- Genetically Engineered Bacterial Biohybrid Microswimmers for Sensing Applications[9]

Bacterial biohybrid microswimmers aim at exploiting the inherent motion capabilities of bacteria (carriers) to transport objects (cargoes) at the microscale. One of the most desired properties of microswimmers is their ability to communicate with their immediate environment by processing the information and producing a useful response. Indeed, bacteria are naturally equipped with such communication skills.[9]
Bacteria have been intensively studied for centuries. Nevertheless, today, a new field of research highlights a previously un-envisioned application of these prokaryotic microorganisms that is currently gathering momentum—their potential for the development of microswimmers. Bacterial biohybrid microswimmers usually consist of one (or many) living bacteria and one (or many) abiotic particles [1]. Depending on the bacteria-to-particle ratio, the hybrid microswimmer can be classified as a Bacteria Bot (1 bacterium:1 cargo) (examples in references [2,3,4]), a Multi Bot (1 bacterium:>1 cargoes) (examples in references [5,6]), or a Bacterial Microsystem (>1 bacteria:1 cargo) (examples in references [7,8]). In these microswimmers, the bacteria usually act as carriers that provide the driving force required to move the inert cargoes from an initial point A to a final point B, i.e., bacterial metabolism hereby provides the chemical energy required for this microscale mechanical transport process. Three critical stages can be identified during the creation of these self-propelled bacterial biohybrid microswimmers—the capture, the delivery, and the release of the cargo (Figure 1). It is important to emphasize that one desired property of these microswimmers is their ability to perceive and react to environmental cues in an autonomous way (Figure 1). All these critical challenges on the developed biohybrid microswimmers are strongly determined by the bacterial characteristics.[9]
The composition of the bacterial surface determines its attachment properties [9,10] and has a direct influence on the initial cargo capture and, potentially, the final cargo release. Gram-positive and Gram-negative bacteria are encased in a multilayered cell envelope with different architectures. In Gram-positive bacteria, the cytoplasmic membrane is surrounded by a thick cell wall of peptidoglycan; in Gram-negative bacteria, the cytoplasmic membrane is surrounded by a thin layer of peptidoglycan and an additional outer membrane called the lipopolysaccharide layer [11]. In both cases, the bacterial surface net charge is predominantly negative, and its electrostatic properties are influenced by the ionization of the functional groups from the macromolecules present in the cell walls and membranes [12]. Surface differences like the length of lipopolysaccharide molecules or the presence/absence of extracellular polymeric substances result in different adhesions of bacteria to inorganic surfaces [13]. Consequently, one approach to improve the attachment of bacteria to their cargo consists of modifying the bacterial surface composition. For example, Gram-negative Escherichia coli cells were modified to express biotin on their surface, thereby allowing the binding to streptavidin-functionalized microparticles [14]. Another approach is to use the natural electronegativity of the cells to bind positively charge molecules. For example, Gram-positive B. subtilis cells were attached to amino-functionalized zeolite L crystals [3].[9]
The delivery of the cargo depends on the type of bacterial locomotion. Three different active movements of bacteria have been used for the development of bacterial biohybrid microswimmers, as reviewed in reference [1]—swimming, swarming, and gliding motilities. Swimming and swarming both depend on the use of flagella [15,16]. Differential flagellation patterns result in different possibilities for movement trajectories like the run-and-tumble swimming of E. coli [17] or the forward, reverse, and turning by buckling of Vibrio alginolyticus [18]. These swimming properties are exploited for the development of biohybrid microswimmers [19]. Furthermore, besides the self-actuated biohybrid microswimmers with uncontrolled motion (due to the action of individual cells in the absence of stimuli [20]), the bacterial taxis mediated by specific receptors and signal transduction pathways can be used to steer the directionality of the cargo transport toward or away from specific stimuli [5,21].[9]
Free-swimming microorganisms
- bacterial flagella
Unicellular microorganisms use cilia or flagella to propel in fluids at the microscale. These elastic paddles exhibit a specific beating pattern to overcome the scallop theorem.16 Although there are thousands of such individual swimming microorganisms, the biomolecular composition and propulsion mechanism of their motor units are surprisingly similar. There is however a sharp distinction between bacterial and eukaryotic flagella (see Fig. 2). The first of these two big groups, bacteria flagella, are connected to the main body of the respective bacterium by a literally rotating molecular joint.32,60–62 Upon rotation, the flagella form a helical shape due to their elasticity which is subject to the anisotropic drag of the fluidic surrounding and leads to a corkscrew-like motion of the bacterium.16,62–64 Usually, a bacterium has multiple flagella which are configured either lophotrichous, i.e., on one side of the bacterium's body, amphitrichous, i.e., on opposite sides of the body, or peritrichous, i.e., all over the bacterium's surface.65 These flagella may rotate individually or form bundles of several flagella that intertwine and rotate together.62,65 If a bacterium has only one flagellum, the configuration is called monotrichous. Most bacteria swim in the so-called run-and-tumble pattern, which describes unidirectional motion periods (i.e., run) that are followed by a stationary state (i.e., tumble) where the bacterium stops to reorient.63 For well-known peritrichous bacteria like Escherichia coli (E. coli), Serratia marcescens (S. marcescens), and Salmonella Typhimurium (S. typhimurium), it was observed that the propelling flagella bundles untangle during this reorientation to allow single rotating flagella to alter the direction of the bacteria's movement.62,63 These three are among the most frequently employed species for bacteria-powered hybrid microswimmers because of their well-known behavior, easy culture conditions, relatively high swimming speeds of roughly 40 μm/s (20 bodylengths per second), chemotaxis capabilities, and genetic alteration possibilities. Other employed bacteria are listed in Table I. A different class of bacteria that proved to be very interesting for hybrid biomicromotors are magnetotactic bacteria (MTB), also listed in Table I. They feature different flagella configurations, e.g., Magnetospirillum gryphiswaldense (M. gryphiswaldense) with two individual amphitrichous flagella, and especially Magnetococcus marinus strains (e.g., MC-1 and MO-1) reach very high speeds of roughly 200 μm/s (100 bodylengths per second), but their most important advantage is the magnetosomes inside their bodies.66,67 These are naturally synthesized magnetite (Fe3O4) nanocrystals that are arranged as a superparamagnetic chain within the bacterium that naturally aligns its magnetic dipole moments to the earth's magnetic field.68,69 This serves as a means for orientation for the bacterium but can be exploited with stronger artificial external magnetic fields to forcefully align and control the magnetotactic bacterium and its motion.[10]
- Eukaryotic flagella
The second big group of flagellated microorganisms are eukaryotic swimmers. Eukaryotic flagella are different from their bacterial counterparts as they are not rotating, but beating in a whiplash fashion.16,70,71 Although the power stroke of the molecular motor unit is actually reciprocal, the elasticity of the flagellum leads to an overhanging, damped wave-like beating pattern that allows non-reciprocal motion in low Reynolds number fluids.16,72 Most prominent examples are mammalian sperm cells that are monoflagellated and have various sizes and swimming speeds depending on the species (see Table I).73–75 Another eukaryote that was employed for hybrid microswimmers is Chlamydomonas Reinhardtii (Chlamydomonas), which is a microalga with two lophotrichous flagella.76 Trypanosomes are eukaryotic parasites that have their flagellum tightly bound to the cell membrane over almost all of its length, which makes the whole body an undulating swimmer that moves with the tail tip in front.77–79 Their distinctive ability to adapt their surface to make them invisible to the immune system in a mammalian host's blood makes them interesting candidates for in vivo hybrid biomicromotors, but the highly dangerous pathogenicity of common trypanosome species prevented the exploration of this possibility so far.80 Microorganisms that employ cilia instead of flagella were also barely used in hybrid approaches. Cilia are structurally similar to flagella, but generally shorter, and are usually distributed all over the surface of the respective microbe, which is called holotrichous arrangement.71,76,81,82 The non-reciprocal wave pattern that is necessary for propulsion thereby does not arise from the beating pattern of individual cilia, but from their concerted beating as a metachronal wave, i.e., like a Mexican wave performed by a sports stadium crowd.83–86 One example that was employed in a hybrid approach is Tetrahymena pyriformis (Tetrahymena), a eukaryotic protozoon that is bigger than most other employed microorganisms, but also significantly faster, with a swimming speed of roughly 500 μm/s (10 bodylengths per second).87 [10]
- amoeboid movement or crawling motion using pseudopodia
There are other unicellular organisms that move without any cilia or flagella. In principle, most cells can migrate by pushing and pulling their bodies by membrane protrusions formed by actin-filaments, so-called pseudopodia.88,89 This crawling motion is called amoeboid movement.90,91 It is not suitable to propel free-swimming micromotors because it is very slow (<1 μm/s) and needs surfaces to crawl along. However, some suspension cells that feature this motion mechanism are nonetheless interesting for hybrid biomicromotor applications. These are mainly blood cells like macrophages and T cells that can be employed to infiltrate solid tumors. This will be discussed further below. Also, red blood cells (RBCs) were employed as hybrid drug carriers.92 They were propelled artificially, by ultrasound, as they naturally only move passively in the flow of blood. Such hybrid biomicromotors that rely on artificial instead of cellular propulsion are not as common as cell-propelled swimmers and will be discussed individually further below as well.[10]
- compression of muscle cells
A final type of cellular motion is the compression of muscle cells (myocytes). Of course, muscle cells do not swim in nature, as they are stationary, but can be employed in a biohybrid approach to move synthetic devices and make them swim. Heart muscle cells of rats (cardiomyocytes) were employed to actuate microtools, but also micromotors like artificial flagella.93,94 For larger devices, skeletal muscle cells and insect dorsal vessel tissue were used, but these are not the focus of this review.95,96 Apart from propulsion, another important aspect of microswimming is taxis. Taxis is the ability to respond to an external stimulus with a change in motion direction.101 For motile microorganisms, this is necessary to find food and purpose.102,103 The most common form of taxis is chemotaxis, i.e., the response to a chemical cue that is present in the surrounding medium.104,105 Practically, all motile microorganisms are capable of some form of chemotactic response, and consequently, many cell-driven hybrid biomicromotors were designed to rely on these capabilities as well, as will be discussed below. Aerotaxis106,107 and pH-taxis108,109 are distinctive forms of chemotaxis that describe the response to oxygen concentration or pH of the liquid medium, respectively. Physical instead of chemical stimuli may also induce tactic behavior in certain microbes, for example, light (phototaxis),110–112 temperature (thermotaxis),113,114 electric fields (galvanotaxis),115 or magnetic fields (magnetotaxis).116–119 Response to mechanical stimuli like liquid flow or to the contact with solid objects can be described as rheotaxis120–122 and thigmotaxis,123,124 respectively. The response in all of these cases can be positive or negative, i.e., the microswimmer may move towards or away from the respective stimulus. An important point here is the difference between klinotactic response and tropism. For example, considering chemotaxis, most microbes have only one sensory capability, i.e., they can sense whether the concentration of a certain chemical increased or decreased at their current location, compared to their previous one, and then decide to alter their movement direction or not.125,126 They can change their direction klinotactically, but they cannot decide on a particular new direction based on their sensing. By contrast, tropism requires more complex sensory organs to go directly up a perceived gradient.127 For example, our eyes allow us to walk directly toward a light source that we see, i.e., phototropically. A phototactic bacterium however would not see the light source, it would have to stop to sense the light intensity in its immediate surroundings, and then, it would move again and stop to sense again until it reaches the point with the highest relative intensity after several directional changes. With the biohybrid approach, we can impose more effective means of taxis artificially, for example, magnetotactic behavior via synthetic magnetic attachments, and we can impose tropism by altering the magnetic field in response to the visual feedback that we receive and judge by ourselves. The microorganisms however still offer the advantage of combining multiple forms of taxis simultaneously, as was pointed out and employed in several hybrid biomicromotor approaches that will be discussed in the main section of this review.101,128 [10]
<<<< TABLE >>>>
Muscle cells Cardiomyocyte148 15 × 100 ca. 5 μN Contraction Tissue (endogenous) aLongitudinal section area of the bulk body (without flagella/cilia). bRoughly averaged swimming speed may vary depending on the liquid medium.
E M Purcell showed that a body has to perform non-reciprocal motion in order to propel itself in a highly viscous environment. The swimmer with one degree of freedom is bound to do reciprocal motion, whereby the center of mass of the swimmer will not be able to propel itself due to the Scallop theorem.[11]

See also
References
- ^ doi:10.3389/fcimb.2021.689197..
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help)CS1 maint: unflagged free DOI (link) Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License - doi:10.1007/s12213-020-00130-7..
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License - doi:10.1002/smll.202007403.)
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help - doi:10.15698/mic2020.03.710.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License - doi:10.1038/s41377-020-00378-5..
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License - ^ doi:10.1002/aisy.201900137..
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License - ^ doi:10.1038/ncomms10598..
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License - doi:10.1088/2516-1091/ab22d5..
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) Material was copied from this source, which is available under a Creative Commons Attribution 3.0 International License
Material was copied from this source, which is available under a Creative Commons Attribution 3.0 International License - ^ doi:10.3390/s20010180.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help)CS1 maint: unflagged free DOI (link) Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License - ^ a b c d Cite error: The named reference
Schwarz2017was invoked but never defined (see the help page). - doi:10.1088/2399-6528/aaa856..
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) Material was copied from this source, which is available under a Creative Commons Attribution 3.0 International License
Material was copied from this source, which is available under a Creative Commons Attribution 3.0 International License - doi:10.1017/jfm.2020.142..
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License
Soil microbiome and virome
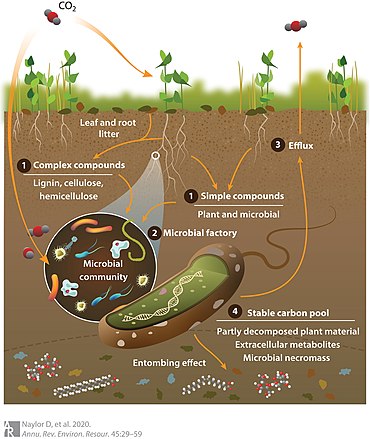
The study of soil viruses, though not new, has languished relative to the study of marine viruses. This is particularly due to challenges associated with separating virions from harboring soils. Generally, three approaches to analyzing soil viruses have been employed: (1) Isolation, to characterize virus genotypes and phenotypes, the primary method used prior to the start of the 21st century. (2) Metagenomics, which has revealed a vast diversity of viruses while also allowing insights into viral community ecology, although with limitations due to DNA from cellular organisms obscuring viral DNA. (3) Viromics (targeted metagenomics of virus-like-particles), which has provided a more focused development of ‘virus-sequence-to-ecology’ pipelines, a result of separation of presumptive virions from cellular organisms prior to DNA extraction. This separation permits greater sequencing emphasis on virus DNA and thereby more targeted molecular and ecological characterization of viruses. Employing viromics to characterize soil systems presents new challenges, however. Ones that only recently are being addressed. Here we provide a guide to implementing these three approaches to studying environmental viruses, highlighting benefits, difficulties, and potential contamination, all toward fostering greater focus on viruses in the study of soil ecology.[3]
Communities of soil microorganisms (soil microbiomes) play a major role in biogeochemical cycles and support of plant growth. Here we focus primarily on the roles that the soil microbiome plays in cycling soil organic carbon and the impact of climate change on the soil carbon cycle. We first discuss current challenges in understanding the roles carried out by highly diverse and heterogeneous soil microbiomes and review existing knowledge gaps in understanding how climate change will impact soil carbon cycling by the soil microbiome. Because soil microbiome stability is a key metric to understand as the climate changes, we discuss different aspects of stability, including resistance, resilience, and functional redundancy.We then review recent research pertaining to the impact of major climate perturbations on the soil microbiome and the functions that they carry out. Finally, we review new experimental methodologies and modeling approaches under development that should facilitate our understanding of the complex nature of the soil microbiome to better predict its future responses to climate change.[2]
New Zealand’s marine coastal ecosystems
- The Importance of Connected Ocean Monitoring Knowledge Systems and Communities - Case studies from Canada and New Zealand
New Zealand’s mangroves
Mangroves are part of the indigenous flora of Aotearoa (New Zealand) and have been part of the natural environment for approximately 19 million years [22]. They are the most southerly growing mangrove ecosystem in the world. The only existing mangrove species in New Zealand is Avicennia marina subsp. australasica, which has existed there for over 11,000 years [23] and currently occupies an area of approximately 177 km2 (data compiled [24,25]). Mangroves range from Cape Reinga in the far North of Northland, to Ohiwa harbour in the Bay of Plenty, on the East Coast and Kawhia harbour on the West coast [26,27,28] (Figure 1). Prior to the definition of ecosystem services, both Kūchler (1972) and Dingwall (1984) recognised that there was no direct utilisation of New Zealand’s temperate mangrove for fuelwood, charcoal, or timber [29,30]. Historically, mangroves or mānawa had previous provisioning services for Māori. They were utilised for their tanning properties, as tools for pounding fern-root, and as dyes for clothes. Post-colonisation, boat-builders used green mangrove wood for shaping the stern and bow [31] (p. 23).[4]
Marine coastal ecosystems
- Sustainable Development Goal 14 - Life Below Water - Towards a Sustainable Ocean
- Governing the Land-Sea Interface to Achieve Sustainable Coastal Development
- Mapping Global Research on Ocean Literacy: Implications for Science, Policy, and the Blue Economy
- Collaborative Automation and IoT Technologies for Coastal Ocean Observing Systems
- Coastal ecosystems - science direct
Overview


"Coastal ecosystems occur where the land meets the sea and that includes a diverse set of habitat types like the mangroves, coral reefs, seagrass beds, estuaries and lagoons, backwaters etc."[6]
"Coastal ecosystems include highly biodiverse marine communities that vary depending on local topography and climate".[7]
"Coastal ecosystems are the unique habitats formed by plants and other organisms that can thrive at the borders between ocean and land, where they must live in saltwater and changing tides. Like forests, many of these coastal ecosystems are full of plants that help regulate the Earth’s temperature. As the plants in these ecosystems grow, they pull carbon out of the air and store it in their tissue, roots, and the soil beneath them. This keeps carbon out of our atmosphere, where, as the greenhouse gas carbon dioxide, it would otherwise trap heat and warm the planet."[8]
"The coastline of the United States is highly populated. Approximately 25 million people live in an area vulnerable to coastal flooding.[1][2] Coastal and ocean activities, such as marine transportation of goods, offshore energy drilling, resource extraction, fish cultivation, recreation, and tourism are integral to the nation's economy, generating 58% of the national gross domestic product (GDP).[2] Coastal areas are also home to species and habitats that provide many benefits to society and natural ecosystems."[9]
"More than 3 billion people rely on the oceans for their livelihoods and more than 350 million jobs are linked to oceans worldwide".[10]
"Which coastal ecosystem is most important for biodiversity? Coral reefs are believed by many to have the highest biodiversity of any ecosystem on the planet—even more than a tropical rainforest. Occupying less than one percent of the ocean floor, coral reefs are home to more than twenty-five percent of marine life".[11]
"Coastal areas help prevent erosion; filter pollutants; and provide food, shelter, breeding areas, and nursery grounds for a wide variety of organisms."[12]

<ref> tag has too many names (see the help page).
Natural coastal ecosystems with connections to the Great Barrier Reef [13]
Ecosystem values
Coastal wetlands, such as seagrass beds, mangrove wetlands, salt marshes, macroalgal, and seaweed beds, shellfish reefs, tidal freshwater wetlands, and coral reefs, are remarkable features of tropical and temperate coastlines. They provide vital services, including coastal protection, fisheries production, blue carbon capture, and pollutant removal and detoxification (Nagelkerken et al., 2015).[14]
Wave attenuation
Coastal vegetation is found around the globe in the form of seagrass fields, kelp forests, salt marshes and mangrove forests.[16] The vegetation across and within these habitats differs significantly, ranging from flexible grasses to rigid shrubs and trees. When vegetation is present on or seaward of the coastline, it interacts with incoming waves.[17][15]
Vegetation contributes to coastal protection by damping incoming waves.[18][19][20] When waves travel over vegetation, energy is dissipated due to the work done by wave forces on plants (Dalrymple et al., 1984). This can significantly reduce wave impact on beaches and hard structures, lowering their construction and maintenance costs (Temmerman et al., 2013; Vuik et al., 2016). Additionally, vegetation reduces storm surge propagation, stabilises shorelines during storms, and contributes to sediment capture, carbon storage and recreational opportunities outside storm events (Bouma et al., 2014; Fagherazzi et al., 2012; Stark et al., 2016; Sutton-Grier et al., 2015; Wamsley et al., 2009).[15]
Stem motion of flexible vegetation can impact wave damping significantly as has been demonstrated in experimental (Luhar et al., 2017; Paul et al., 2016; Riffe et al., 2011) and computational studies (Luhar and Nepf, 2016; Mullarney and Henderson, 2010). Vegetation species are broadly classified as rigid or flexible. Rigid vegetation, like woody shrubs, does not move over a wave cycle, whereas flexible vegetation, like thin grass, sways as its rigidity is insufficient to resist stem bending. The excursion of flexible species increases when its flexural rigidity decreases or wave forces increase (Luhar et al., 2017). As stem bending increases, the plant frontal area and the relative velocity between water and stem decrease (Méndez et al., 1999; Paul et al., 2016). Both limit the wave forces on the plant and may reduce wave damping by up to 50–70% (Luhar et al., 2017; Mullarney and Henderson, 2010; Riffe et al., 2011; van Veelen et al., 2020). However, as the interaction between plant motion and wave forces is reciprocal, quantifying wave damping over flexible species poses a challenge.[15]
Computational models can be a valuable tool to quantify wave damping for variable vegetation properties. For rigid vegetation, Dalrymple et al. (1984) simplified vegetation fields to arrays of rigid cylinders on a flat bottom and assumed validity of linear wave theory to model damping of monochromatic waves. Under these assumptions, they demonstrated that wave damping is dominated by drag, and wave heights reduce proportionally to the distance travelled over vegetation. Using the same modelling framework, Mendez and Losada (2004) proposed to calibrate the drag coefficient to include the effect of stem motion. Their model was successfully applied in field (Bradley and Houser, 2009; Garzon et al., 2019; Jadhav et al., 2013) and flume studies (Anderson and Smith, 2014; Augustin et al., 2009; Koftis et al., 2013; [19] [20] van Veelen et al., 2020), but the calibrated drag coefficients vary widely between plant species and test conditions,[16] Vuik et al., 2016), and when vegetation conditions change (Schulze et al., 2019). Thus, site-specific calibration for each coastal habitat is required.[15]
Seascape ecology

There is a growing demand for the sea space due to an expansion of traditional maritime sectors and emerging marine uses, such as wind farms and mariculture. Marine spatial planning (MSP) is a means to allocate space to all these activities, while avoiding conflict and creating synergies (Douvere, 2008). MSP can be defined as the process to spatially and temporally allocate human activities in marine areas such that environmental, economic, and social objectives can be achieved (Ehler and Douvere, 2009). MSP thus needs to strike a balance between increasing maritime uses (fostered by the EU Blue Growth strategy (EC, 2012), for example) and the sustainable development of these activities within the natural limits (i.e., the carrying capacity) of the ecosystem (Hassler et al., 2019). The latter emphasizes the ecosystem approach to MSP that considers the entire ecosystem. The ecosystem approach encompasses the view that present and future generations should be able to continue using the goods and services provided by the sea (UNCED, 1992; UNEP, 2011). This links the approach to the ecosystem service (ES) concept. ES can be defined as the contributions of ecosystems to human wellbeing and can be divided into provisioning, regulating and maintenance, and cultural services (Haines-Young and Potschin, 2010a). In the EU, the ES concept has made its way into relevant, marine-related legislation, such as the Marine Strategy Framework Directive (MSFD) (EC, 2008) and the Directive establishing a framework for maritime spatial planning (MSP Directive) (EC, 2014).[21]
Vegetated coastal habitats

Causes of habitat degradation and disturbance
CMS are in decline globally due to a variety of anthropogenic disturbances (Table 1). While coastal habitats experience a wide variety of threats, below we highlight some of the most urgent and increasing pressures on these ecosystems: rising global temperatures, sea-level rise (SLR), deforestation and dredging, pollution, and invasive species.[23]
Table 1. Anthropogenic disturbances affecting mangrove, seagrass, and coral reef ecosystems. The source of each disturbance is noted alongside the results of disturbance, and potential protection resulting from the interaction between mangroves, seagrasses, and reefs.[23]
| Land-sea ecosystem | Disturbance | Result | Protection from mangrove-seagrass-reef interactions |
|---|---|---|---|
| Mangroves | Clear cutting | * Aquaculture development is a major driver of mangrove loss (Ellegaard et al., 2014). • Average loss of 0.18% per year of mangroves in southeast Asia (Richards and Friess, 2016). • Cleared vs non-cleared mangrove sites show shifts in fish assemblages (Shinnaka et al., 2007) • Rearrangement in species assemblages: cleared sites show more zooplanktivorous species compared to a greater component of benthic crustacean feeders found at mangrove sites (Shinnaka et al., 2007). |
• Blue carbon markets from seagrasses and mangroves, and tourism in coral reefs and mangroves, can provide alternative sources of income to disincentivize clear-cutting (Spalding et al., 2017). • Payments for blue carbon can result in greater profits than converting mangroves to shrimp farms (Yee, 2010). |
| Sea level rise | • Low-lying islands are at the highest risk of SLR due to lack of sediment deposition (Gilman et al., 2007). • Mangroves cannot migrate in areas with strong coastal development. • In the absence of coastal squeeze (development barriers), mangroves may “back-step” into landward habitats (Saintilan et al., 2014, Di Nitto et al., 2014). • Mangroves fringing larger landmasses and areas with significant river outputs are less vulnerable to SLR (McKee and Vervaeke, 2009). • Mangroves at low tidal ranges are unlikely to grow at a sufficient rate, whereas mangroves at high tidal ranges are already positioned farther inland (Ward et al., 2016). |
• Wave buffering from coral reefs and seagrasses can increase sediment trapping and accretion in coastal habitats (Alongi, 2008). • Blue carbon storage potential of mangroves and seagrasses help offset carbon emissions (cause of SLR) (Alongi, 2012). | |
| Increased temperatures | • Warmer climates may allow mangroves to expand at latitudinal maxima (Alongi, 2015, Kelleway et al., 2017, Osland et al., 2017). However, poleward expansion may be inhibited by low habitat availability or dispersal barriers (Hickey et al., 2017). • Rates of leaf photosynthesis for most species peak at temperatures at or below 30 °C, and leaf CO2 assimilation rates of many species decline as temperature increases from 33 °C to 35 °C (Alongi, 2015). |
• Blue carbon storage potential of mangroves and seagrasses help offset carbon emissions (cause of increased global and regional temperatures)(Alongi, 2012; Greiner et al., 2013). | |
| Extreme storms | • Hurricanes can alter mangrove stem size-frequency distributions and relative abundance, density (Smith et al., 2009). • Some mangroves recover while others are converted to mud flats, with basin (rather than island) mangroves being the most vulnerable (Smith et al., 2009). |
• Wave buffering from coral reefs and seagrasses mitigates damage from hurricanes and tropical storms, preventing phase shifts to mud flats (Alongi, 2008). • Blue carbon storage potential of mangroves and seagrasses help offset carbon emissions (contributor of extreme climatic events) (Alongi, 2012; Greiner et al., 2013). | |
| Dredging | • Fill material after mangrove clearing is often dredged from nearby reefs (Macintyre et al., 2009). | • Economic value of coastal ecosystems due to storm protection can be greater than economic benefits from degrading the system (Grabowski et al., 2012). • Blue carbon markets from seagrasses and mangroves, and tourism in coral reefs and mangroves, can provide alternative sources of income to disincentivize dredging (Yee, 2010, Spalding et al., 2017). | |
| Marine and terrestrial debris | • Mangroves can act as sinks for marine and terrestrial debris, mediating the impacts of marine and terrestrial debris on adjacent systems (Martin et al., 2019). | ||
| Pollutants | • High inputs of untreated domestic sewage, storm water run-off, shipping effluent, and heavy metal contamination inhibit mangrove regeneration and growth (Defew et al., 2005). • Oil spills drive mangrove toxicity, algal blooms, and conversion to mud flats (Santos et al., 2012, Duke, 2016). |
• Seagrass communities reduce the amount of pathogens and bacteria in the water where adjacent systems show less impact from disease (Lamb et al., 2017, Sullivan et al., 2018). | |
| Invasive species | • Introduction of two non-native mangrove species (Brugeria gymnorrhiza and Lumnitzera racemosa) in southern Florida have modified the structure and function of these mangrove forests (Carlton, 1989, Fourqurean et al., 2010). | ||
| Seagrass | Removal | • Coastal development is a frequent cause of removal. • Area reduction of ~110 km2 y-1since 1980; 29% of known seagrass beds have disappeared since they were initially recorded in 1879 (Waycott et al., 2009). |
• Hotels and coastal businesses may consider seagrasses an eyesore or nuisance. The need to preserve coral reefs (a tourism draw) can help justify seagrass protection (Daby, 2003). • Economic value of coastal ecosystems due to storm protection can be greater than economic benefits from degrading the system (Grabowski et al., 2012). • Blue carbon storage potential may disincentivize removal. |
| Increased turbidity | • Higher levels of turbidity results in lower irradiance, photosynthesis, and shoot density and growth. This drives lower sequestration of atochthonous blue carbon by seagrasses (Mazarrasa et al., 2018). • Turbidity can increase the accumulation of allochthonous carbon and fine sediment particles (Mazarrasa et al., 2018). |
• Mangroves prevent sediment deposition, reducing turbidity in seagrasses. | |
| Sea level rise | • Sea level rise compresses seagrass habitats if not offset by sediment accretion, improvement in water clarity, and/or managed retreat from the coast (freeing up seagrass habitat). SLR may be responsible for a predicted 17% loss of seagrass habitat from 2000 to 2100 (Saunders et al., 2013). | • Mangroves prevent sediment deposition, thereby improving water clarity. Seagrass persistence depends on water clarity under SLR; seagrasses may also migrate into mangrove habitats. • Wave buffering from coral reefs can increase sediment trapping and accretion in seagrasses (Guerra-Vargas et al., 2020). | |
| Increased temperatures | • Increase in water temperatures will depress photosynthetic rates and increase the amount of respiration (Short and Neckles, 1999). • Habitat contraction has already been noted and populations will begin to see drastic habitat incursion and species rearrangements as temperatures increase (Short and Neckles, 1999, Fraser et al., 2015). |
• Blue carbon storage potential of mangroves and seagrasses help offset carbon emissions (cause of increased global and regional temperatures)(Alongi, 2012; Greiner et al., 2013). | |
| Extreme storms | • Strong rainfall events could lead to mortality, and/or sediment accretion and seagrass growth. However, as sediment increases from rainfall and storm events, photosynthesis decreases and seagrasses experience widespread defoliation (Macreadie et al., 2019). | • Wave buffering from coral reefs mitigates damage from hurricanes and tropical storms (Ferrario et al., 2014, Guannel et al., 2016). • Mangroves prevent sediment deposition. • Blue carbon storage potential of mangroves and seagrasses help offset carbon emissions (contributor of extreme climatic events) (Alongi, 2012; Osland et al., 2018). | |
| Invasive species | • At least 28 non-native species have become established in seagrass beds worldwide, of which 64% have documented or inferred negative effects (Orth et al., 2006) | • Reducing additional pressure on native species is increasingly important. Mangrove-seagrass-reef interactions support nurseries and trophic redundancy for native species (Saenger et al., 2013). | |
| Disease | • Increased rates of disease are associated with warming temperatures (Sullivan et al., 2018). • Unicellular, mold-like protist Labyrinthula zosterae causes leaf cellular and chloroplast necrosis (Bull et al., 2012). |
||
| Nutrients and sediment pollution | • Runoff of artificial nitrogen fertilizer results in blooms of plankton in the ocean. Plankton blooms result in increased bacterial activity, creating oxygen-poor environments not conducive to healthy ecosystem functioning (Corredor and Morell, 1994, Ramanan et al., 2016). | • Mangroves can denitrify sewage effluent (Tam and Wong, 1999, Ouyang and Guo, 2016). • Mangroves bioaccumulate heavy metals, for example, by precipitating sulfide minerals; absorbing and dissolving iron oxides; and binding soils (Attri and Kerkar, 2011). Mangroves thus prevent contaminant flows to ocean habitats. | |
| Coral reefs | Example | Example | Example |
| Example | Example | Example | |
| Example | Example | Example | |
| Example | Example | Example | |
| Example | Example | Example | |
| Example | Example | Example | |
| Example | Example | Example | |
| Example | Example | Example |
Coral reefs Dredging
•
Sediments accumulate on living substrates.
• Increases in turbidity and reduced light irradiance reduces coral calcification rates and can increase the prevalence of diseases (Pollock et al., 2014).
• Payments for ecosystem services (fishing, tourism, blue carbon) in mangroves, seagrasses, and reefs can provide alternative economic benefits, i.e., incentives against dredging (McKee and Vervaeke, 2009, Lau, 2013).
Pollutants • Oil spills, heavy metal contamination, excessive nutrients, and marine debris may cause long-term disturbances (Todd et al., 2010, Smith, 2018, Mearns et al., 2019).
• Mangroves and seagrasses can absorb excess nitrogen (Adame et al., 2010).
• Mangroves and seagrasses prevent heavy metal transport to coral reefs (Smith, 2018, Gopi et al., 2020).
Invasive species • Non-native algae may compete with corals for substrate, and invasive fish (e.g., lionfish) may cause fish and hydrophyte extinctions and decrease biodiversity (Carlton, 1989).
• Mangroves and seagrasses increase fish diversity, and may create ecological niche space for species threatened by invasives.
Disease • Coral disease contributes to large scale coral mortality worldwide.
• Disease occurrence can be intensified by other stressors such as increased temperature and pollutants (Bruno et al., 2007).
• Mangroves can potentially reduce thermal and photic stress on corals (Rogers, 2017), which may help coral combat disease.
• Seagrass meadows reduce the relative abundance of potential bacterial pathogens up to 50%, where adjacent systems benefit from reduction in disease (Lamb et al., 2017).
Sea level rise • Sea level rise may increase turbidity and sediment levels and change wave dynamics, which decrease photosynthesis due to reduced light, and result in structural stress on coral reefs (Storlazzi et al., 2011, Baldock et al., 2014).
• Mangroves and seagrasses prevent sediment deposition
• Blue carbon storage potential of mangroves and seagrasses help offset carbon emissions (cause of SLR) (Alongi, 2012; Greiner et al., 2013).
Increased temperatures • Increases in mean sea surface temperatures and marine heatwaves has led to worldwide coral bleaching events (Brown, 1997, Burke et al., 2011).
• In addition to severe mortality, bleaching events may lead to poleward range shifts in coral recruits and dominance of stress-tolerant species (Loya et al., 2001, Price et al., 2019).
• Mangroves provide lower light environments for corals, reducing bleaching stress.
• In particular, mangroves are alternative habitats (refuges) for coral species that thrive in low pH and high turbidity environments (Yates et al., 2014, Camp et al., 2016, Camp et al., 2019).
Extreme storms • In some cases, dramatic freshwater inputs into reef environments by storms or flooding events can negatively impact coral health and in extreme cases cause widespread mortality, particularly in larvae and juvenile corals. Effects are mediated by coral life history and morphology, site variables like depth, and other factors (Rogers, 1993).
• Mangroves enhance fish populations, dramatically increasing chances of ecosystem recovery (Mumby and Hastings, 2007).
Overfishing • Reduction in herbivores increases turf and macroalgae on coral reefs, often causing phase shifts from coral- to algal-dominated systems.
• Overfishing exacerbates coral mortality after bleaching events; healthy fish populations predict reef recovery (Graham et al., 2007, McClanahan et al., 2011).
• Mangroves and seagrasses serve as nursery habitats for diverse fish, buffering some negative effects of overfishing in reefs (Wakwabi, 1999, Crona and Rönnbäck, 2005, Saenger et al., 2013).
References
- PMID 33873756.
- ^ doi:10.1146/annurev-environ-012320-082720..
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License - doi:10.3390/soilsystems4020023..
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help)CS1 maint: unflagged free DOI (link) Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License - ^ doi:10.3390/resources7010023..
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help)CS1 maint: unflagged free DOI (link) Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License - ^ Land Information New Zealand (LINZ). Accessed: 15 April 2017
- ^ [1]
- ^ Coastal ecosystem facts
- ^ Coastal Ecosystems and Climate Change MIT
- ^ Climate Impacts on Coastal Areas EPA
- ^ Oceans Economy and Ecosystem services: sustainable fisheries and coastal tourism United Nations Conference on Trade and Development
- ^ Coral Reef Biodiversity Coral Reef Alliance
- ^ Ripple Effects: Population and Coastal Regions Population Reference Bureau
- ISBN 978 1 922126 21 4
- ^ Cite error: The named reference
Waltham2020was invoked but never defined (see the help page). - ^ S2CID 229402284..
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License - ^ a b {{cite journal |doi = 10.1142/9789813231283_0001}
- doi:10.1016/j.geomorph.2017.11.001.)
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help - doi:10.1016/j.coastaleng.2015.09.011.)
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help - ^ doi:10.1016/j.coastaleng.2015.09.011.)
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help - ^ doi:10.1038/NGEO2251.)
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help - ^ doi:10.1016/j.ocecoaman.2019.105071..
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License - doi:10.3389/fmars.2019.00014..
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License - ^ ISSN 2351-9894..
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License
Seagrass

Mangroves
Overview

- Dahdouh-Guebas F. (Ed.) (2021) World Mangroves database. Accessed: 5 October 2021. doi:10.14284/460.
Mangroves are a phylogenetically diverse group of species comprising mostly woody trees and shrubs, but also including some palms and ferns.[2][3]
Mangroves are a taxonomically diverse group of ±70 tree, shrub, and fern species (in at least 25 genera and 19 families) that grow in anoxic and saline peaty soils on sheltered, tropical coasts. Mangroves share a suite of genetic, morphological, physiological, and functional traits that provide one of the most convincing cases for convergent evolution among diverse taxa in response to similar environmental constraints.[4][5][6] Mangroves can be found throughout the tropics, with representatives of the major mangrove genera Rhizophora and Avicennia present in both the Indo-West Pacific (IWP) and the Atlantic, Caribbean, and Eastern Pacific (ACEP) realms.[7][5] Mangrove species diversity is much lower in the ACEP, where it reaches a maximum of 8–9 species at any given site, than in the IWP, where 30 or more species from the regional pool of at least 46 can co-occur.[7] At least 16% of mangrove species worldwide are currently considered to be of conservation concern.[4][8]
Where they grow, mangroves can form dense, often monospecific stands whose species composition is determined in large part by tidal elevation.[9] Mangroves are thought to be one of the few good examples of foundation tree species in the tropics.[10][11] They create habitats for many terrestrial, intertidal, and marine species, stabilize shorelines, and modulate nutrient cycling and energy flow through the forests they define.[9] Mangrove forests have some of the highest reported net primary productivity of any ecosystem on the planet, and their loss or deliberate removal leads to rapid build-up of acid sulfides in the soil, increased shoreline erosion and sedimentation onto offshore coral reefs, and collapse of intertidal food webs and inshore fisheries.[9][8]
A recent fine-scale analysis of global mangrove forest cover yielded an estimate of ≈ 84,000km2 spread across 105 countries (including special administrative areas and French overseas provinces).
"Mangal has become the accepted name for the community; mangroves for the constituent plants".[16]{rn|xi}}
Because mangroves live in the intertidal zone, rising sea levels put mangroves in the front line of pending changes.[16]xi
PARAPHRASE => "Mangroves were certainly known to the ancient peoples,
- WHAT IS A MANGROVE?
FROM: American Museum of Natural History
"If you've ever spent time by the sea in a tropical place, you've probably noticed distinctive trees that rise from a tangle of roots wriggling out of the mud. These are mangroves—shrub and tree species that live along shores, rivers, and estuaries in the tropics and subtropics. Mangroves are remarkably tough. Most live on muddy soil, but some also grow on sand, peat, and coral rock. They live in water up to 100 times saltier than most other plants can tolerate. They thrive despite twice-daily flooding by ocean tides; even if this water were fresh, the flooding alone would drown most trees. Growing where land and water meet, mangroves bear the brunt of ocean-borne storms and hurricanes."[20]
- One Ingenious Plant
"How do mangroves survive under such hostile conditions? A remarkable set of evolutionary adaptations makes it possible. These amazing trees and shrubs:"[20]
- cope with salt: "Saltwater can kill plants, so mangroves must extract freshwater from the seawater that surrounds them. Many mangrove species survive by filtering out as much as 90 percent of the salt found in seawater as it enters their roots. Some species excrete salt through glands in their leaves. These leaves, which are covered with dried salt crystals, taste salty if you lick them. A third strategy used by some mangrove species is to concentrate salt in older leaves or bark. When the leaves drop or the bark sheds, the stored salt goes with them."[20]
- hoard fresh water: "Like desert plants, mangroves store fresh water in thick succulent leaves. A waxy coating on the leaves of some mangrove species seals in water and minimizes evaporation. Small hairs on the leaves of other species deflect wind and sunlight, which reduces water loss through the tiny openings where gases enter and exit during photosynthesis. On some mangroves species, these tiny openings are below the leaf's surface, away from the drying wind and sun."[20]
- breathe in a variety of ways: "Some mangroves grow pencil-like roots that stick up out of the dense, wet ground like snorkels. These breathing tubes, called pneumatophores, allow mangroves to cope with daily flooding by the tides. Pneumatophores take in oxygen from the air unless they're clogged or submerged for too long."[20]
- Roots That Multitask
"Root systems that arch high over the water are a distinctive feature of many mangrove species. These aerial roots take several forms. Some are stilt roots that branch and loop off the trunk and lower branches. Others are wide, wavy plank roots that extend away from the trunk. Aerial roots broaden the base of the tree and, like flying buttresses on medieval cathedrals, stabilize the shallow root system in the soft, loose soil. In addition to providing structural support, aerial roots play an important part in providing oxygen for respiration.
- Ready-to-Roll Seeds
"The mangroves' niche between land and sea has led to unique methods of reproduction. Seed pods germinate while on the tree, so they are ready to take root when they drop. If a seed falls in the water during high tide, it can float and take root once it finds solid ground. If a sprout falls during low tide, it can quickly establish itself in the soft soil of tidal mudflats before the next tide comes in. A vigorous seed may grow up to two feet (about 0.6 m) in its first year. Roots arch from the seedling to anchor it in the mud. Some tree species form long, spear-shaped stems and roots while still attached to the parent plant. After being nourished by the parent tree for one to three years, these sprouts may break off. Some take root nearby while others fall into the water and are carried away to distant shores."[20]
- A World Traveler
"Botanists believe that mangroves originated in Southeast Asia, but ocean currents have since dispersed them to India, Africa, Australia, and the Americas. As Alfredo Quarto, the head of the Mangrove Action Project, puts it, “Over the millions of years since they've been in existence, mangroves have essentially set up shop around the world.” The fruits, seeds, and seedlings of all mangrove plants can float, and they have been known to bob along for more than a year before taking root. In buoyant seawater, a seedling lies flat and floats fast. But when it approaches fresher, brackish water—ideal conditions for mangroves—the seedling turns vertical so its roots point downward. After lodging in the mud, the seedling quickly sends additional roots into the soil. Within 10 years, as those roots spread and sprout, a single seedling can give rise to an entire thicket. It's not just trees but the land itself that increases. Mud collects around the tangled mangrove roots, and shallow mudflats build up. From the journey of a single seed a rich ecosystem may be born."[20]
FROM: How to Identify the Three Types of Mangroves...
There are over 50 different species of mangroves found around the world
Mangroves can live where others would never make it! They can do this with some pretty unique adaptations. Normally, too much salt water would kill a plant, just like a human who drinks it. However, mangroves have adapted a unique filtration system to remove most of the salt from the water it takes in. They can also save water in their leaves like desert plants to use later on. Even further, some mangroves have special roots that stick up out of the water to help with gas exchange.
Because mangroves have to deal with the tides and waves, it's important that they get anchored into the sediment so they don't get pushed around. Because of this, they are very important land stabilizers. Their extensive root systems also provide habitats for many different animal species.
Red Mangroves If you've seen a mangrove with its roots sticking into the ocean water, you were probably looking at a red mangrove or Rhizophora mangle, named for their red tinted roots. These are the most well-known mangroves because they are the most easily seen.
At the top, red mangroves look like a typical tree with green leaves and a trunk, but when you look further down, you'll likely see some roots branching off the trunk that reach into the water. These are called prop roots and help keep the plant stable. They also provide oxygen for the rest of the plant.
These roots have frequent branching and end up looking like a jumbled mess. This is one of the species that's heavily monitored in the United States because those messy roots help prevent soil erosion.
If you need help remembering this type, think red, red. . . tiny head in reference to the shape on the leaves!
Black Mangroves If you travel a little further inland, you will come across the black mangrove or Avicennia germinans. These plants grow in very wet soil that isn't heavily oxygenated, which is why their roots grow straight up into the air. These roots are called pneumatophores and look like tiny snorkels that help with gas exchange.
Try tasting the back of a black mangrove leaf and you will notice it tastes salty and often has a whitish tint. This is how black mangroves adapt to live in the saltwater—by releasing excess salt onto its leaves!
To remember this type, think black, black. . . lick the back.
White Mangroves Even further inland, you will encounter the white mangrove or Laguncularia racemosa, which looks much more like your typical tree compared to the black and red mangroves. These mangroves like to live on more solid ground but they still get inundated with saltwater from time to time. They usually do not have funky roots like the others but rather typical underground ones.
White mangroves have glands called nectaries at the base of their leaves that produce a sweet secretion that often attracts ants. They also have beautiful small white and green flowers that bloom during spring and summer.
The rhyme for this tree goes white, white. . . sweet delight.
Buttonwood Mangroves Buttonwood mangroves or Conocarpus erectus grow much further inland compared to the rest, and some say they are not true mangroves because of this. The flowers of this tree look like buttons and get grouped together like a cone.
These mangroves are able to withstand a lot, which is why they are often used for landscapes. There are two types of buttonwoods: green and silver. Both have pointed leaves with glands that remove salt.
Fossils


"Mangroves have a long fossil history, which includes traces of distinctive mangrove roots, fruits, and pollen. Pollen fossilizes particularly well, and is distinctive enough for identification to genus, sometimes even to species level. There are, of course, many problems in interpreting fossil evidence. Not all present-day members of the family Rhizophoraceae, for example, are mangroves, so fossil evidence of the existence of a member of this family is not necessarily evidence for the existence of mangroves. Ideally, corroborative evidence of a mangrove environment is desirable, such as fossil molluscs identifiable with modern mangrove residents. Conversely, of course, a fossil may not be recognized as mangrove if it belongs to a taxonomic group that is now extinct, or which has no modern mangrove representatives".[21]: 196
"The fossil evidence, supplemented by genetic comparisons between living mangrove species, suggests the possible course of mangrove evolution, dispersal, and diversification. Fossils interpreted as mangroves have been reported from the early Cretaceous era (Figure 10.4), or even earlier, but most are doubtful. Most mangrove taxa probably appeared first in the late Cretaceous, possibly between 100 and 80 Mya (million years ago), along the shores of Tethys. Nypa pollen appears by 69 Mya, in the late Cretaceous of Brazil, and by the Eocene it is found in the Caribbean, South America, Europe, Asia, and Australia. The earliest reliable mangrove fossil, other than pollen, is of fruit of the palm Nypa, in the early Paleocene. By the Miocene Nypa appears to have retracted to Asia, and, currently, apart from deliberate reintroduction in the Caribbean and West Africa, the single species N. fruticans is limited to Sri Lanka, S.E. Asia, the Philippines, Australia, and New Guinea (Figure 10.5) (Duke 1995; Plaziat 1995; Plaziat et al. 2001)".[21]: 196
- The oldest known fossils of mangrove palm date to 75 million years ago.[22]
- While only one
- The giant seed ferns, Weichselia reticulata. The mangrove ecosystem it inhabited was situated along the southern shore of the Tethys Sea.[24]
A world without mangroves?
- "A World Without Mangroves?"[25]
- Mangroves give cause for conservation optimism, for now[26]
- Conservation Optimism
- Public Perceptions of Mangrove Forests Matter for Their Conservation[27]
Evolution
Genetic divergence of mangrove lineages from terrestrial relatives, in combination with fossil evidence, suggests mangrove diversity is limited by evolutionary transition into the stressful marine environment, and the number of mangrove lineages has increased steadily over the Tertiary with little global extinction.[28]
"The 'vicariance hypothesis' asserts that mangrove taxa evolved around the Tethys Sea during the Late Cretaceous, and regional species diversity resulted from in situ diversification after continental drift."[29]
- "Biogeomorphic evolution and expansion of mangrove forests in New Zealand's sediment-rich estuarine systems". )
- "Evolution and paleobiogeography of mangroves". )
Isotopes

Taxonomy
PARAPHRASE: "The vegetation of mangrove forests is loosely classified as "true mangroves" or "mangrove associates". True mangroves are woody plants, facultative or obligate halophytes (Wang et al. 2011). "True mangroves" are defined by P. B. Tomlinson (2016) as plant species that 1) occur only in mangrove forests and are not found in terrestrial communities; 2) play a major role in the structure of the mangrove community, sometimes forming pure stands; 3) have morphological specialisations to the mangrove environment; 4) have some mechanism for salt exclusion.[16]
Other notable specialisations of mangrove plants include: aerial roots to counteract the anaerobic sediments, support structures such as buttresses and above-ground roots, low water potentials and high intracellular salt concentrations, salt-excretion through leaves and buoyant, viviparous propagules".[30][31]
"This dataset contains traits for 55 species of "true mangroves". To standardise the spelling of species' names, The Plant List (2013) was followed. Some species listed below are currently considered as synonyms in The Plant List (e.g. Avicennia alba is currently a synonym of Avicennia marina). However, they were chosen to be included under the names given by the authors to allow the tracking of the original information. All records of Ceriops tagal var. australis were included as Ceriops australis, and Ceriops tagal var. tagal was included as Ceriops tagal following Ballment et al. (1988)."[31]
"Mangrove species are classified as true mangroves and mangrove associates."[32]
Mangrove environments in the Eastern Hemisphere harbour six times as many species of trees and shrubs as do mangroves in the New World.
True mangroves
| True mangroves (major components or strict mangroves) | ||||
|---|---|---|---|---|
| Following Tomlinson, 2016, the following 35 species are the true mangroves, contained in 5 families and 9 genera [16]: 29–30 Included on green backgrounds are annotations about the genera made by Tomlinson | ||||
| Family | Genus | Mangrove species | Common name | |
| Arecaceae | Monotypic subfamily within the family | |||
| Nypa | Nypa fruticans | Mangrove palm | 
| |
Avicenniaceae (disputed) |
Old monogeneric family, now subsumed in Acanthaceae, but clearly isolated | |||
| Avicennia | Avicennia alba | 
| ||
| Avicennia balanophora | ||||
| Avicennia bicolor | ||||
| Avicennia integra | ||||
| Avicennia marina | grey mangrove (subspecies: australasica, eucalyptifolia, rumphiana) |
|||
| Avicennia officinalis | Indian mangrove | |||
| Avicennia germinans | black mangrove | |||
| Avicennia schaueriana | ||||
Avicennia tonduzii
|
||||
| Combretaceae | Tribe Lagunculariae (including Macropteranthes = non-mangrove) | |||
| Laguncularia | Laguncularia racemosa
|
white mangrove | 
| |
| Lumnitzera | Lumnitzera racemosa | white-flowered black mangrove | ||
| Lumnitzera littorea | ||||
| Rhizophoraceae | Rhizophoraceae collectively form the tribe Rhizophorae, a monotypic group, within the otherwise terrestrial family | |||
| Bruguiera | Bruguiera cylindrica | |||
| Bruguiera exaristata | rib-fruited mangrove | |||
| Bruguiera gymnorhiza | oriental mangrove | 
| ||
| Bruguiera hainesii | ||||
| Bruguiera parviflora | ||||
| Bruguiera sexangula | upriver orange mangrove | |||
| Ceriops | Ceriops australis | yellow mangrove | 
| |
| Ceriops tagal | spurred mangrove | 
| ||
| Kandelia | Kandelia candel | |||
| Kandelia obovata | ||||
| Rhizophora | Rhizophora apiculata | |||
| Rhizophora harrisonii | ||||
| Rhizophora mangle | red mangrove | |||
| Rhizophora mucronata | Asiatic mangrove | 
| ||
| Rhizophora racemosa | ||||
Rhizophora samoensis
|
Samoan mangrove | |||
| Rhizophora stylosa | spotted mangrove, | |||
Rhizophora x lamarckii
|
||||
| Lythraceae | Sonneratia | Sonneratia alba | 
| |
Sonneratia apetala
|
||||
| Sonneratia caseolaris | ||||
| Sonneratia ovata | ||||
Sonneratia griffithii
|
||||
Minor components
| Minor components | ||||
|---|---|---|---|---|
| Tomlinson, 2016, lists about 19 species as minor mangrove components, contained in 10 families and 11 genera [16]: 29–30 Included on green backgrounds are annotations about the genera made by Tomlinson | ||||
| Family | Genus | Species | Common name | |
| Euphorbiaceae | This genus includes about 35 non-mangrove taxa | |||
| Excoecaria | Excoecaria agallocha | milky mangrove, blind-your-eye mangrove and river poison tree | 
| |
| Lythraceae | Genus distinct in the family | |||
| Pemphis | Pemphis acidula | bantigue or mentigi | ||
| Malvaceae | Formerly in Bombacaceae, now an isolated genus in subfamily Bombacoideeae | |||
| Camptostemon | Camptostemon schultzii
|
kapok mangrove | 
| |
Camptostemon philippinense
|
||||
| Meliaceae | Genus of 3 species, one non-mangrove, forms tribe Xylocarpaeae with Carapa, a non–mangrove | |||
| Xylocarpus | Xylocarpus granatum | 
| ||
| Xylocarpus moluccensis | ||||
| Myrtaceae | An isolated genus in the family | |||
| Osbornia | Osbornia octodonta
|
mangrove myrtle | ||
Pellicieraceae
|
Monotypic genus and family of uncertain phylogenetic position | |||
| Pelliciera | Pelliciera rhizophorae,
|
tea mangrove | ||
| Plumbaginaceae | Isolated genus, at times segregated as family Aegialitidaceae
| |||
| Aegialitis | Aegialitis annulata
|
club mangrove | ||
| Aegialitis rotundifolia | ||||
| Primulaceae | Formerly an isolated genus in Myrsinaceae
| |||
| Aegiceras | Aegiceras corniculatum | black mangrove, river mangrove or khalsi | ||
Aegiceras floridum
|
||||
| Pteridaceae | A fern somewhat isolated in its family | |||
| Acrostichum | Acrostichum aureum | golden leather fern, swamp fern or mangrove fern | ||
| Acrostichum speciosum | mangrove fern | |||
| Rubiaceae | A genus isolated in the family | |||
| Scyphiphora | Scyphiphora hydrophylacea
|
nilad | ||
Mangrove associates
Trees
-
High tide
-
Rhizophora sp.
-
Mature Avicennia marina
-
Young Rhizophora mangle L. tree growing on a beach
Soil
Galleries


- fruit and flowers
-
Rhizophora mucronata or red mangrove flowers
-
Mangrove flowers, possibly Sonneratia alba
-
Tall-stilt mangrove (Rhizophora apiculata) flower
-
Mangrove rubbervine, Rhabdadenia biflora
-
Flower of the oriental mangrove,Bruguiera gymnorrhiza
-
Red mangrove, Rhizophora mangle flowers
-
Bruguiera gymnorrhiza
-
fruit of the white mangrove, Avicennia marina
- mangrove propagules and pneumatophores
-
Baby red mangrove, Rhizophora mangle
-
Red mangrove propagules
-
Propagules are used by the mangroves to breathe since normal roots are underwater most of the time
-
Black mangrove pneumatophores
- mangroves
-
Mangrove architecture
-
Mangroves, Yucatan
-
Mangrove roots
-
Mangrove roots
-
Mangroves along a lagoon
-
Mangrove tree stabilises its base
-
Rhizophora mangle (red mangrove) Bahamas
-
Magrove roots provide habitat for numerous marine animals (NOAA)
-
Mangrove roots hanging loose during a low tide
-
Rhizophora mangle roots
-
White mangrove roots
-
Mangrove roots at low tide
-
Mangrove roots



![A typical square-rigged caravel (Livro das Armadas)]]](http://upload.wikimedia.org/wikipedia/commons/thumb/9/9c/Caravela_de_armada_of_Joao_Serrao.jpg/285px-Caravela_de_armada_of_Joao_Serrao.jpg)


































































































































![Brown pelican]] in a mangrove swamp](http://upload.wikimedia.org/wikipedia/commons/thumb/6/6f/Brown_pelican_in_a_mangrove_swamp.jpg/360px-Brown_pelican_in_a_mangrove_swamp.jpg)













































![Interfaces between coastline salt water and fresh water [5]](http://upload.wikimedia.org/wikipedia/commons/thumb/0/0d/Interfaces_between_coastline_salt_water_and_fresh_water.jpg/335px-Interfaces_between_coastline_salt_water_and_fresh_water.jpg)
![Pattern and causes of marsh expansion into a forest [5]](http://upload.wikimedia.org/wikipedia/commons/thumb/8/8b/Pattern_and_causes_of_marsh_expansion_into_a_forest.jpg/668px-Pattern_and_causes_of_marsh_expansion_into_a_forest.jpg)
![Model of ecological ratchet [5]](http://upload.wikimedia.org/wikipedia/commons/thumb/5/55/Proposed_conceptual_model_of_ecological_ratchet.jpg/197px-Proposed_conceptual_model_of_ecological_ratchet.jpg)
![Ecological ratchet model of marsh migration in a forest [5]](http://upload.wikimedia.org/wikipedia/commons/thumb/b/b8/Ecological_ratchet_model_of_marsh_migration_in_a_forest.jpg/373px-Ecological_ratchet_model_of_marsh_migration_in_a_forest.jpg)