5-HT2C receptor agonist
5-HT2C receptor agonists are a class of drugs that activate
The 5-HT2C receptors are one of three subtypes that belong to the serotonin 5-HT2 receptor subfamily along with 5-HT2A and 5-HT2B receptors. The development of 5-HT2C agonists has been a major obstacle, because of severe side effects due to a lack of selectivity over 5-HT2A and 5-HT2B receptors. Activation of 5-HT2A receptors can induce hallucinations, and the activation of 5-HT2B receptors has been implicated in cardiac valvular insufficiency and possibly in pulmonary hypertension.[6][7]
Discovery
In the late 1960s, non-selective serotonin
History
The 5-HT2c receptor agonist
In 1994, sales of the combination drug
Dexfenfluramine inhibits serotonin reuptake, stimulating the release of serotonin. In 1996, dexfenfluramine became the first long-term treatment anti-obesity medication approved in the US; adverse effects observed during clinical trials included dry mouth, diarrhea and drowsiness. In the mid-1990s the US FDA approved dexfenfluramine as a weight loss drug. After several reports of adverse cardiovascular effects, the FDA banned dexfenfluramine in 1997.[12][17][18]
It appears that the 5-HT2B receptors, expressed in cardiac valves, are responsible for the valvulopathies reported from the use of fenfluramine and dexfenfluramine.[19]
The serotonin receptor agonist mCPP has a significant affinity for 5-HT2C receptors. mCPP patients experience multiple side effects due to non-selectivity over 5-HT2A and 5-HT2B receptors. The absence of the hypophagic (reduced food consumption) effect of mCPP in 5-HT2C receptor knockout mice suggests that this effect is mediated through 5-HT2C receptor activation. Repeated administration of mCPP to humans might result in decreased food intake and weight loss. mCPP is used as a prototype research tool for drug discovery of selective 5-HT2C receptor agonists.[20][21][22]
Mechanism of action
This section may be too technical for most readers to understand. (January 2016) |
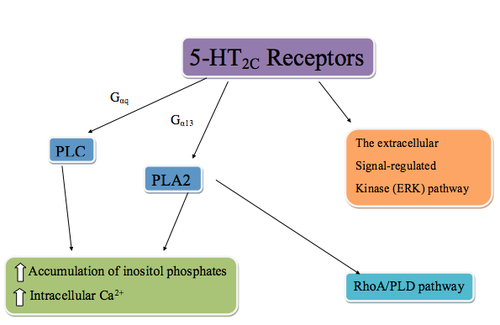
The
5-HT2C receptors are located only within the CNS, where they can be found in several locations. The highest density of receptor expression is within the
Binding
The 5-HT2C receptors and ligand binding

5-HT2 receptors are
5-HT2C and 5-HT2A receptors have a similar
The 5-HT2C receptors are the only G-protein coupled receptors known to undergo a post-transcriptional process of RNA editing. The 5-HT2C receptor gene is found on the
Serotonin binding to 5-HT2C
Serotonin is an

Pharmacophore
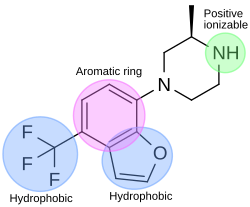
In the drug discovery process of a 5-HT2C agonist, a pharmacophore module has been used to discover novel 5-HT2C receptor ligands. The pharmacophore has four features; one aromatic ring, two hydrophobic features and one positive ionizable feature. Figure 4 shows an example of a compound that fits the agonist pharmacophore perfectly. The nitrogen atom of piperazine fits the positive ionizable feature, the benzofuran part fits the aromatic ring and one hydrophobic, and the trifluoromethane part fits another hydrophobic feature of the pharmacophore.[32]
Structure-activity relationships
In a

A series of 3-benzazepine derivatives, such as Lorcaserin (Figure 6) have been evaluated for their potency and selectivity for the 5-HT2C receptors. Lorcaserin is a very potent agonist, but the potency is dependent on the presence of a chloro substituent in position 8.[7][33][34]
Arylpiperazine-containing compounds such as mCPP (Figure 7), show good potency toward the 5-HT2C receptors, but do not have sufficient selectivity for the 5-HT2C receptors over the other two receptor subtypes. Many derivatives have been examined in an attempt to increase the selectivity. Derivatives lacking the arylpiperazine core, such as 4-aryl-1,2,3,6-tetrahydropyridinum chlorine analogues, are more favorable for potency and selectivity over the other two receptors (Figure 7).[35]
Functional selectivity
In 2016 the discovery of novel G protein biased 5-HT2C receptor agonists was published.[36]
Drug development
Obesity
Obesity is a global epidemic health problem and has received considerable attention as a major public hazard. Obesity is a chronic pathological and costly disease of abnormal or excessive fat accumulation in the body.[37]
Studies indicate that 5-HT2C receptor activation will regulate appetite and food consumption, most likely by promoting satiety through appetite suppression by activation of 5-HT2C. Consequently, selective agents with high affinity for this receptor over 5-HT2B and 5-HT22A are being developed for the treatment of obesity.[38][6]
Pumosetrag was the example set by Mitsubishi Chemical Corporation.
Lorcaserin
Lorcaserin is a full agonist for 5-HT2C and 5-HT2B receptors and
Psychiatric disorders
Serotonin plays an important role in numerous physiological conditions. 5-HT2 receptor antagonists have long been known, but recently 5-HT2 receptor agonists are becoming promising agents in the development for new
A 5-HT2C agonist may be expected to reduce positive symptoms of schizophrenia by reducing dopamine release in the
Vabicaserin and aripiprazole
Vabicaserin has a high affinity for 5-HT2C receptors and low affinity for 5-HT2B and 5-HT2A receptors. Vabicaserin is a full agonist with approximately 4-fold greater selectivity for 5-HT2C over these related receptors, in terms of binding affinity. Vabicacserin is a full agonist in stimulating the 5-HT2C receptor; it was discovered when a class of tetrahydroquinoline-fused diazepines were being researched as possible potent 5-HT2C receptor agonists.[43]
As of 2012, vabicaserin is in clinical trials for the treatment of schizophrenia. Long-term administration of vabicaserin significantly decreased the number of spontaneously active mesocorticolimbic dopamine neurons without affecting
Aripiprazole is also a mild partial agonist of 5HT2C receptor.
Sexual dysfunction
Activation of 5-HT2C receptor subtype has been reported to mediate numerous effects, such as penile erection.
Urinary incontinence
Serotonin plays a key role in mechanisms involved in
Current status
Many
| Compound name | Chemical name | Mode of action | Company | Phase of development | Indication | Reference |
|---|---|---|---|---|---|---|
| PRX-00933 | N/A | 5-HT2C agonist | Proximager | Phase III (2011) | Obesity and diabetes | [51] |
| Vabicaserin | (9aR,12aS)-4,5,6,7,9,9a,10,11,12,12a- decahydrocyclopenta[c] [1,4]diazepino[6,7, 1-ij]quinoline | 5-HT2C agonist | Pfizer | Phase I (February, 28th,2012) | Schizophrenia | [52] |
| Lorcaserin | 1R)-8-chloro-2,3,4,5-tetrahydro-1-methyl- 1H-3-benzazepine | 5-HT2C agonist | Arena Pharmaceuticals | FDA approved (June 27, 2012) | Obesity | [53] |
See also
- 5-HT receptor
- 5-HT2 receptor
- 5-HT2C receptor
- Serotonin receptor agonist
- Serotonin antagonist
- Serotonin
- Lorcaserin
- Vabicaserin
References
- PMID 18600561.
- PMID 11082448.
- ^ PMID 20006638.
- ISBN 978-1-60761-940-6.
- S2CID 10831801.
- ^ PMID 21267355.
- ^ S2CID 20924745.
- S2CID 27485889.
- ^ PMID 20202843.
- PMID 19029184.
- ^ PMID 21190985.
- ^ PMID 9731094.
- PMID 8541174.
- S2CID 22100586.
- S2CID 2830353.
- S2CID 27455236.
- PMID 8910095.
- S2CID 45849415.
- PMID 10617681.
- S2CID 4368727.
- PMID 3290473.
- S2CID 7125577.
- S2CID 33007.
- PMID 12650852.
- PMID 15993039.
- PMID 14623358.
- S2CID 25596946.
- ^ PMID 16269190.
- PMID 10220565.
- S2CID 30964513.
- PMID 18499489.
- ^ PMID 19464901.
- PMID 15713408.
- PMID 20022752.
- PMID 22381048.
- PMID 27726356.
- S2CID 4415341.
- PMID 14709324.
- S2CID 221625777.
- PMID 11082448.
- S2CID 22518050.
- ^ PMID 20852829.
- S2CID 26937933. Archived from the original(PDF) on 2019-03-03.
- S2CID 793693.
- PMID 1535317.
- S2CID 13007057.
- PMID 18582863.
- PMID 7938165.
- PMID 16600805.
- ^ PMID 21195614.
- ^ Proximagen group plc. "Annual Report & Accounts 2011: Working together" (PDF). Proximagen. Archived from the original (PDF) on 16 September 2012. Retrieved 26 September 2012.
- ^ Pfizer. "Pfizer Pipeline" (PDF). Pfizer. Retrieved 26 September 2012.
- ^ FDA. "BELVIQ". Food and Drug Administration. Retrieved 26 September 2012.

![Pyrazolo[3,4-d]pyrimidine core](http://upload.wikimedia.org/wikipedia/commons/thumb/8/8d/5-HT2C-hit-general.svg/157px-5-HT2C-hit-general.svg.png)