Alachlor
| |

| |
| Names | |
|---|---|
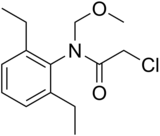
| Preferred IUPAC name
2-Chloro-N-(2,6-diethylphenyl)-N-(methoxymethyl)acetamide | |
| Identifiers | |
3D model (
JSmol ) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
ECHA InfoCard
|
100.036.448 |
| KEGG | |
PubChem CID
|
|
RTECS number
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C14H20ClNO2 | |
| Molar mass | 269.767 g/mol |
| Appearance | cream-coloured solid |
| Odor | odorless |
| Density | 1.133 g/cm3 |
| Melting point | 39.5 °C (103.1 °F; 312.6 K) |
| Boiling point | 404 °C (759 °F; 677 K) |
| 0.0242 g/100 mL | |
| Solubility | soluble in ethyl ether, ethyl acetate
|
| Vapor pressure | 1x106 mmHg |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Toxic |
| Flash point | 198 °C (388 °F; 471 K) |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
930 mg/kg |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |

Alachlor is an herbicide from the chloroacetanilide family. It is an odorless, white solid. The greatest use of alachlor is for control of annual grasses and broadleaf weeds in crops. Use of alachlor is illegal in the European Union[1] and no products containing alachlor are currently registered in the United States.[2]
Its mode of action is elongase
Uses
The largest use of alachlor is as a herbicide for control of annual grasses and broadleaf weeds in crops, primarily on corn, sorghum, and soybeans.[4]
Application details
Alachlor mixes well with other herbicides. It is marketed in mixed formulations with
It is most commonly available as microgranules containing 15% active ingredients (AI), or emulsifiable concentrate containing 480 g/ litre of AI. Homologuation in Europe requires a maximum dose of 2,400 g per hectare of AI, or 5 litres/hectare of emulsifiable concentrate or 17 kg/ha of microgranules. The products are applied as either pre-drilling, soil incorporated or pre-emergence.[7]
Safety
The
The EPA cited the following long-term effects for exposures at levels above the MCL in drinking water exposed to runoff from herbicide used on row crops: slight skin and eye irritation; at lifetime exposure to levels above the MCL: potential damage to liver, kidney, spleen; lining of nose and eyelids; cancer. The major source of environmental release of alachlor is through its manufacture and use as a herbicide. Alachlor was detected in rural domestic well water by EPA's National Survey of Pesticides in Drinking Water Wells. EPA's Pesticides in Ground Water Database reports detections of alachlor in ground water at concentrations above the MCL in at least 15 U.S. states.[9]
Alachlor is a controlled substance under Australian law and is listed as a Schedule 7 (Dangerous Poison) substance. Access, use and storage are strictly controlled under state and territory law.[10] Since 2006, use of alachlor as a herbicide has been banned in the European Union.[1]
In "a judgment that could lend weight to other health claims against pesticides," in January, 2012 a French court found
Environmental fate
Alachlor exhibits moderate
Alachlor is often used in high school chemistry classrooms as a reactant in demonstrations such as the combustion of magnesium. Alachlor can be used as a substitution for methane gas in such an experiment when gas is not available.[15]
See also
References
- ^ ISBN 1437708811. Retrieved 4 September 2015.
- ^ "Chemical Name". iaspub.epa.gov. Retrieved 2018-12-01.
- ^
- ISBN 9789290580478. Retrieved 6 February 2016.
- ^ Cornell Herbicide Profile
- ^ a b "EXTOXNET PIP - ALACHLOR". orst.edu. Retrieved 17 May 2015.
- ^ "e-phy". Ministère de l'agriculture, de l'agroalimentaire (in French). Retrieved 30 April 2013.
- ^ US EPA Consumer Factsheet
- ^ "Consumer Factsheet on: ALACHLOR" (PDF). National Primary Drinking Water Regulations. United States Environmental Protection Agency (EPA. Retrieved 4 September 2015.
- ^ "Poisons Standard 2013". comlaw.gov.au. Retrieved 17 May 2015.
- ^ Douet, Marion (13 February 2012). "Monsanto guilty of chemical poisoning in France". Reuters. Retrieved 30 April 2013.
- ^ "French court confirms Monsanto liable in chemical poisoning case". Reuters. 2015-09-11. Retrieved 2015-09-15.
- ISBN 1-891276-33-6.
- ^ Xu, J., J. W. Stucki, J. Wu, J. Kostka, and G. K. Sims. 2001. Fate of atrazine and alachlor in redox-treated ferruginous smectite. Environmental Toxicology & Chemistry 20: 2717-2724.
- ^ Kappa, Bromide 2013. Substitutions for methane gas in high school combustion demonstrations.
