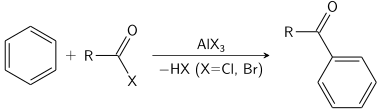Electrophilic aromatic substitution
Electrophilic aromatic substitution (SEAr) is an
Illustrative reactions
The most widely practised example of this reaction is the ethylation of benzene.
Approximately 24,700,000 tons were produced in 1999. gives the corresponding aryl halides. This reaction is typically catalyzed by the corresponding iron or aluminum trihalide.
The
Reaction mechanism
The overall reaction mechanism, denoted by the
Occasionally, other electrofuges (groups that can leave without their electron pair) beside H+ will depart to reestablish aromaticity; these species include silyl groups (as SiR3+), the carboxy group (as CO2 + H+), the iodo group (as I+), and tertiary alkyl groups like t-butyl (as R+). The capacity of these types of substituents to leave is sometimes exploited synthetically, particularly the case of replacement of silyl by another functional group (ipso attack). However, the loss of groups like iodo or alkyl is more often an undesired side reaction.
Effect of substituent groups

Both the
Reaction rate
Substituents can generally be divided into two classes regarding electrophilic substitution: activating and deactivating towards the aromatic ring. Activating substituents or
.The extra electron density delivered into the ring by the substituent is not distributed evenly over the entire ring but is concentrated on atoms 2, 4 and 6, so activating substituents are also ortho/para directors (see below).
On the other hand,
Ortho/para directors
Groups with
In addition to the increased nucleophilic nature of the original ring, when the electrophile attacks the ortho and para positions of aniline, the
When the electrophile attacks the meta position, the nitrogen atom cannot donate electron density to the pi system, giving only three resonance contributors. This reasoning is consistent with low yields of meta-substituted product.
Other substituents, such as the
Directed ortho metalation is a special type of EAS with special ortho directors.
Meta directors
Non-halogen groups with atoms that are more electronegative than carbon, such as a carboxylic acid group (-CO2H), withdraw substantial electron density from the pi system. These groups are strongly
The reaction is also much slower (a relative reaction rate of 6×10−8 compared to benzene) because the ring is less nucleophilic.
Reaction on pyridine
Compared to benzene, the rate of electrophilic substitution on pyridine is much slower, due to the higher electronegativity of the nitrogen atom. Additionally, the nitrogen in pyridine easily gets a positive charge either by protonation (from nitration or sulfonation) or Lewis acids (such as AlCl3) used to catalyze the reaction. This makes the reaction even slower by having adjacent formal charges on carbon and nitrogen or 2 formal charges on a localised atom. Doing an electrophilic substitution directly in pyridine is nearly impossible.
In order to do the reaction, they can be made by 2 possible reactions, which are both indirect.
One possible way to do a substitution on pyridine is nucleophilic aromatic substitution. Even with no catalysts, the nitrogen atom, being electronegative, can hold the negative charge by itself. Another way is to do an oxidation before the electrophilic substitution. This makes pyridine N-oxide, which due to the negative oxygen atom, makes the reaction faster than pyridine, and even benzene. The oxide then can be reduced to the substituted pyridine.
Ipso attack
The attachment of an entering group to a position in an aromatic compound already carrying a substituent group (other than hydrogen). The entering group may displace that substituent group but may also itself be expelled or migrate to another position in a subsequent step. The term 'ipso-substitution' is not used, since it is synonymous with substitution.[4] A classic example is the reaction of salicylic acid with a mixture of nitric and sulfuric acid to form picric acid. The nitration of the 2 position involves the loss of CO2 as the leaving group. Desulfonation in which a sulfonyl group is substituted by a proton is a common example. See also Hayashi rearrangement. In aromatics substituted by silicon, the silicon reacts by ipso substitution.
Five membered heterocycles
Compared to benzene, furans, thiophenes, and pyrroles are more susceptible to electrophilic attack. These compounds all contain an atom with an unshared pair of electrons (oxygen, sulfur, or nitrogen) as a member of the aromatic ring, which substantially stabilizes the cationic intermediate. Examples of electrophilic substitutions to pyrrole are the Pictet–Spengler reaction and the Bischler–Napieralski reaction.
Asymmetric electrophilic aromatic substitution
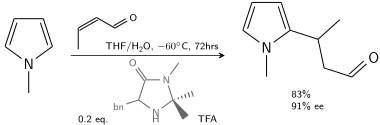
Electrophilic aromatic substitutions with
In another alkylation N-methylpyrrole reacts with crotonaldehyde catalyzed by trifluoroacetic acid modified with a chiral imidazolidinone:[7]
In the presence of 10–20 % chiral catalyst, 80–90% ee is achievable.
Other reactions
- Other reactions that follow an electrophilic aromatic substitution pattern are a group of aromatic formylation reactions including the Gattermann Koch reaction and the Reimer–Tiemann reaction.
- Other electrophiles are aromatic carbonyl groups in the Pechmann condensation, hydroxycarbenium ion in the Blanc chloromethylation via an intermediate (hydroxymethyl)arene (benzyl alcohol), chloryl cation (ClO3+) for electrophilic perchlorylation.
- In the multistep Lehmstedt–Tanasescu reaction, one of the electrophiles is a N-nitroso intermediate.
- In the Tscherniac–Einhorn reaction (named after Joseph Tscherniac and Alfred Einhorn) the electrophile is a N-methanol derivative of an amide[9][10]
See also
References
- ISBN 978-0-471-72091-1
- ISSN 0040-4039.
- ^ Verfahren zur Darstellung von Benzylphtalimiden Joseph Tscherniac German Patent 1902, DE-134,979