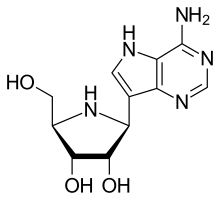
Galidesivir
 | |
| Legal status | |
|---|---|
| Legal status | |
| Identifiers | |
| |
JSmol) | |
| |
| |
Galidesivir (BCX4430, immucillin-A) is an
Ebola virus disease and Marburg virus disease, as well as Zika virus.[3] Currently, galidesivir is under phase 1 human trial in Brazil for coronavirus.[4]
It also shows
Ebola virus epidemic in West Africa.[6]
Galidesivir later showed efficacy against Zika virus in a mouse model.[7]
Galidesivir abrogates viremia in Zika virus–infected rhesus Macaques.[8]
Galidesivir is one of several antiviral drugs being tested for
coronavirus disease 2019.[9]
On April 9, 2020, BioCryst opened enrollment into a randomized, double-blind, placebo-controlled clinical trial to assess the safety, clinical impact and antiviral effects of galidesivir in patients with COVID-19.[4]
See also
- Atoltivimab/maftivimab/odesivimab
- Bemnifosbuvir
- Brincidofovir
- Coronavir
- 3-Deazaneplanocin A
- Favipiravir
- FGI-106
- GS-441524
- JK-05
- Lopinavir/ritonavir
- Lamivudine
- Ansuvimab
- MK-608
- Molnupiravir
- Nelfinavir
- Oseltamivir
- Nirmatrelvir/ritonavir
- Peramivir
- Remdesivir
- Ribavirin
- Ensitrelvir
- TKM-Ebola
- Triazavirin
- Umifenovir
- ZMapp
References
- ^ PMID 24590073.
- PMID 24111876.
- ^ BioCryst Pharmaceuticals, Inc. (June 10, 2020). "Galidesivir Stops Zika Viral Replication in Primate Model". GlobeNewswire News Room (Press release).
- ^ a b Clinical trial number NCT03891420 for "A Study to Evaluate the Safety, Pharmacokinetics and Antiviral Effects of Galidesivir in Yellow Fever or COVID-19" at ClinicalTrials.gov
- PMID 29864447.
- ^ Rodgers P (8 April 2014). "BioWar Lab Helping To Develop Treatment For Ebola". Forbes Magazine.
- PMID 27838352.
- PMID 32522808.
- ^ Duddu P (19 February 2020). "Coronavirus outbreak: Vaccines/drugs in the pipeline for Covid-19". clinicaltrialsarena.com. Archived from the original on 19 February 2020. Retrieved 19 February 2020.
