Glatiramer acetate
 | |
| Clinical data | |
|---|---|
| Trade names | Copaxone,[1] Glatopa,[2] Brabio |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a603016 |
| License data |
|
| Pregnancy category |
|
Subcutaneous injection | |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
ECHA InfoCard | 100.248.824 |
| Chemical and physical data | |
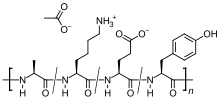
| Formula | C25H45N5O13 |
| Molar mass | 623.657 g·mol−1 |
| | |
Glatiramer acetate (also known as Copolymer 1, Cop-1), sold under the brand name Copaxone among others, is an
It is a mixture of random-sized peptides that are composed of the four
It is on the World Health Organization's List of Essential Medicines.[6]
History
Glatiramer acetate was originally discovered at the
Medical uses
Glatiramer acetate is indicated for the treatment of relapsing forms of multiple sclerosis (MS), to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults.[1]
A 2010 Cochrane review concluded that glatiramer acetate had partial efficacy in "relapse-related clinical outcomes" but no effect on progression of the disease.[10] As a result, it is approved by the FDA for reducing the frequency of relapses, but not for reducing the progression of disability.[1][2]
A 15-year followup of the original trial compared patients who continued with glatiramer to patients who dropped out of the trial. Patients with glatiramer had reduced relapse rates, and decreased disability progression and transition to secondary progressive MS, compared to patients who did not continue glatiramer. However, the two groups were not necessarily comparable, as it was no longer a randomized trial. There were no long-term safety issues.[11]
Adverse effects

Side effects may include a lump at the injection site (injection site reaction) in approximately 30% of users, and aches, fever, chills (flu-like symptoms) in approximately 10% of users.[12] Side effect symptoms are generally mild in nature. A reaction that involves flushing, shortness of breath, anxiety and rapid heartbeat has been reported soon after injection in up to 5% of patients (usually after inadvertently injecting directly into a vein). These side effects subside within thirty minutes. Over time, a visible dent at a repeat-injection site can occur due to the local destruction of fat tissue, known as lipoatrophy, that may develop.
More serious side effects have been reported for glatiramer acetate, according to the FDA's prescribing label, these include serious side effects to the cardiovascular, digestive (including the liver), hematopoietic, lymphatic, musculoskeletal, nervous, respiratory, and urogenital systems as well as special senses (in particular the eyes). Metabolic and nutritional disorders have also been reported; however a link between glatiramer acetate and these adverse effects has not been established.[1][2]
It may also cause
Mechanism of action
Glatiramer acetate is a random polymer (average molecular mass 6.4
The integrity of the blood–brain barrier, however, is not appreciably affected by glatiramer acetate, at least not in the early stages of treatment. Glatiramer acetate has been shown in clinical trials to reduce the number and severity of multiple sclerosis exacerbations.[16]
Society and culture
Marketing
Glatiramer acetate has been approved for marketing in numerous countries worldwide, including the
Patent status
Novartis subsidiary Sandoz has marketed Glatopa since 2015, a generic version of the original Teva 20 mg formulation that requires daily injection.[23]
Teva developed a long-acting 40 mg formulation, marketed since 2015, which reduced required injections to three per week.[24] In October 2017, the FDA approved a generic version, which is manufactured in India by Natco Pharma, and imported and sold by Dutch firm Mylan.[25][26] In February 2018, Sandoz received FDA approval for their generic version.[27] In parallel with the development and approval processes, the generic competitors have disputed Teva's newer patents, any of which if upheld, would prevent marketing of long-acting generics.[28]
While the
References
- ^ a b c d e f g h "Copaxone- glatiramer acetate injection, solution". DailyMed. 23 July 2020. Retrieved 11 November 2020.
- ^ a b c d e f g "Glatopa- glatiramer acetate injection, solution". DailyMed. 31 July 2020. Retrieved 11 November 2020.
- ^ "Neurological therapies". Health Canada. 9 May 2018. Retrieved 13 April 2024.
- ^ "Brabio 20 mg/mL Solution for Injection, Pre-filled Syringe - Summary of Product Characteristics (SmPC)". (emc). Retrieved 11 November 2020.
- ^ "Copaxone 20 mg/ml solution for injection in pre-filled syringe - Summary of Product Characteristics (SmPC)". (emc). 29 September 2020. Retrieved 11 November 2020.
- hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.
- PMID 3302705.
- S2CID 28895177.
- S2CID 35614752.
- PMID 20464733.
- PMID 20106943.
- ^ "Copaxone". MediGuard.
- ISBN 978-0-7234-3665-2.
- ^ Arnon R, Sela M (1999). "The chemistry of the Copaxone drug" (PDF). Chem. Israel. 1: 12–17. Archived from the original (PDF) on 2003-09-07.
- ^ https://go.drugbank.com/drugs/DB05259; Glatiramer Mechanism of Action
- ^ "Copaxone". All About Multiple Sclerosis.
- S2CID 30186027.
- .
- ^ "Copaxone". CenterWatch.
- ^ "Teva's Copaxone approved in UK". The Pharma Letter.
- ^ "Glatiramer Acetate". Tofigh Daru Research and Engineering Company. Archived from the original on 2018-10-21. Retrieved 2017-02-01.
- ^ Isayev S, Jafarov T (1 May 2012). "Iran to manufacture multiple sclerosis cure". Trend News Agency.
- ^ "Sandoz announces US launch of Glatopa". Novartis. 2015. Archived from the original on 2018-02-03. Retrieved 2017-02-21.
- ^ Silva P (9 October 2015). "New 3-Times-Per-Week Regimen For Teva's Copaxone". Multiple Sclerosis News Today.
- ^ Erman M, Grover D (3 October 2017). "Mylan surges, Teva slumps after FDA okays Copaxone copy". Reuters. Retrieved 4 October 2017.
- ^ "NATCO's marketing partner Mylan receives final approval of generic glatiramer acetate, for both 20 mg/mL and 40 mg/mL versions". NATCO Pharma (India). 3 October 2017. Archived from the original on 10 January 2019. Retrieved 4 October 2017.>
- ^ "Sandoz announces US FDA approval and launch of Glatopa 40 mg/mL". Novartis International AG. February 13, 2018. Retrieved May 10, 2018.
- ^ "Teva's Copaxone still growing despite patent risks". BioPharmaDive.
- ^ Helfand C. "Copaxone". FiercePharma.
- ^ Decker S (1 September 2016). "Teva loses decision on validity of 302 copaxone patent". Bloomberg Markets.
- ^ Decker S, Flanagan C, Benmeleh Y (30 January 2017). "Teva loses ruling invalidating patents on copaxone drug". Bloomberg Markets.
- ^ "Teva loses patent ruling". Briefcase. The Philadelphia Inquirer. Bloomberg News. September 2, 2017. p. A12. Retrieved June 23, 2018 – via Newspapers.com (Publisher Extra).
- ^ "U.S. appeals court upholds ruling that canceled Teva Copaxone patents". Reuters. October 12, 2018. Retrieved October 12, 2018.
- ^ "In Re: Copaxone Consolidated Cases" (PDF). United States Court of Appeals for the Federal Circuit. October 12, 2018. Retrieved October 12, 2018.
