Vitamin D
| Vitamin D | |
|---|---|
vitamin D receptor | |
| Clinical data | |
| Drugs.com | MedFacts Natural Products |
| External links | |
| MeSH | D014807 |
| Legal status | |
| In Wikidata | |
Vitamin D is a group of fat-soluble secosteroids responsible for increasing intestinal absorption of calcium, magnesium, and phosphate, and for many other biological effects.[1][2][3] In humans, the most important compounds in this group are vitamin D3 (cholecalciferol) and vitamin D2 (ergocalciferol).[2][3][4]
The major natural source of vitamin D is synthesis of cholecalciferol in the lower layers of the epidermis of the skin, through a photochemical reaction with Ultraviolet B (UV-B) radiation from sun exposure or UV-B lamps.[1] Cholecalciferol and ergocalciferol can be ingested from the diet and supplements.[1][2] Only a few foods, such as the flesh of fatty fish, naturally contain significant amounts of vitamin D.[2][5] In the U.S. and other countries, cow's milk and plant-derived milk substitutes are fortified with vitamin D, as are many breakfast cereals.[1] Mushrooms exposed to ultraviolet light contribute useful amounts of vitamin D2.[2][6] Dietary recommendations typically assume that all of a person's vitamin D is taken by mouth, because sun exposure in the population is variable and recommendations about the amount of sun exposure that is safe are uncertain in view of the associated risks of skin cancer.[2]
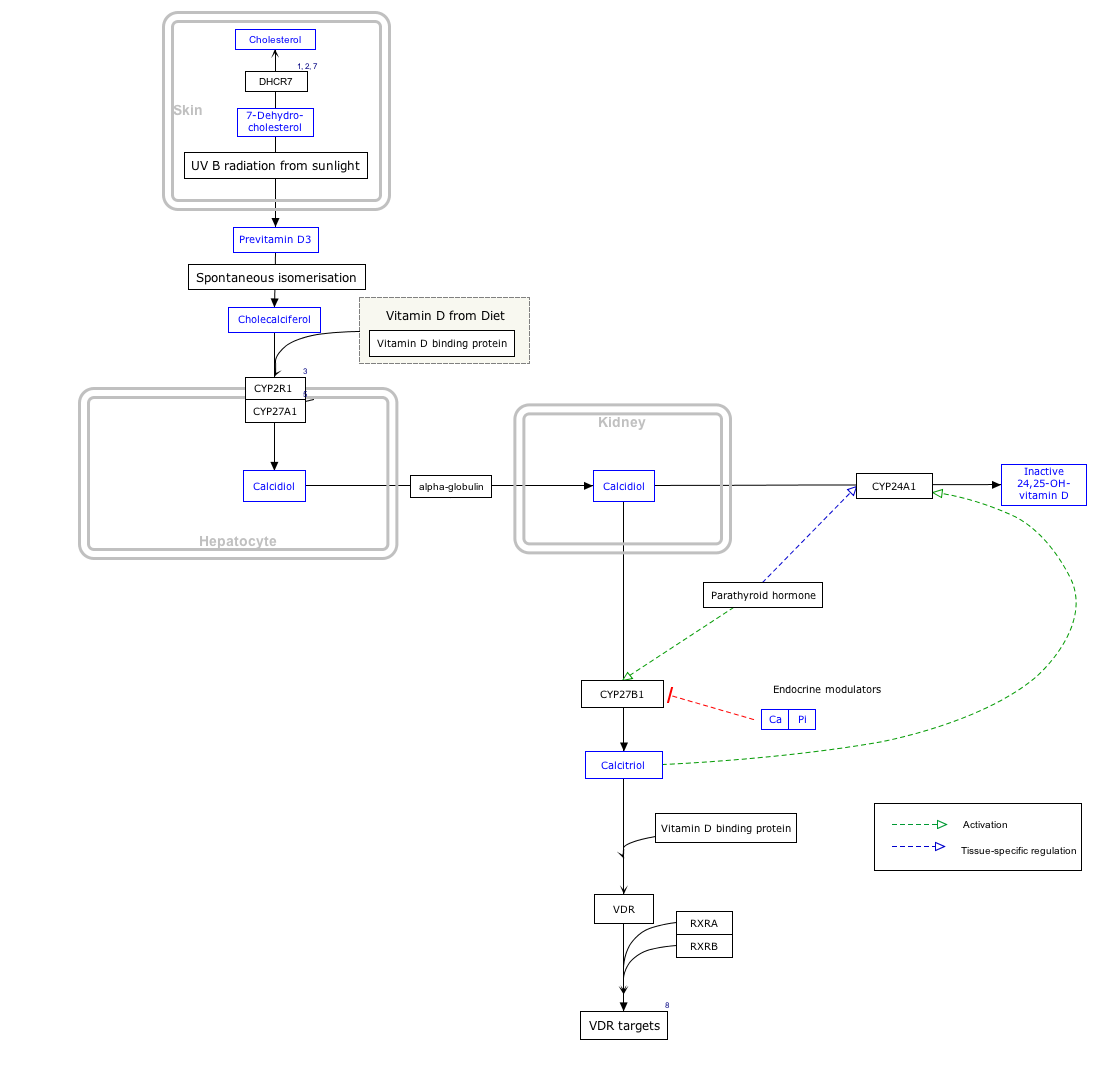
Vitamin D from the diet, or from skin synthesis, is biologically inactive. It is activated by two protein enzyme hydroxylation steps, the first in the liver and the second in the kidneys.[1][4] Because vitamin D can be synthesized in adequate amounts by most mammals if they get enough sunlight, it is not essential and therefore is technically not a vitamin.[3] Instead it can be considered a hormone, with activation of the vitamin D pro-hormone resulting in the active form, calcitriol, which then produces effects via a nuclear receptor in multiple locations.[3]
Cholecalciferol is converted in the liver to calcifediol (25-hydroxycholecalciferol); ergocalciferol is converted to 25-hydroxyergocalciferol.[1] These two vitamin D metabolites (called 25-hydroxyvitamin D or 25(OH)D) are measured in serum to determine a person's vitamin D status.[7][8] Calcifediol is further hydroxylated by the kidneys and some of the immune system cells to form calcitriol (1,25-dihydroxycholecalciferol), the biologically active form of vitamin D.[9][10] Calcitriol circulates as a hormone in the blood, having a major role regulating the concentration of calcium and phosphate, and promoting the healthy growth and remodeling of bone.[1] Calcitriol also has other effects, including some on cell growth, neuromuscular and immune functions, and reduction of inflammation.[2]
Vitamin D has a significant role in
Types
| Name | Chemical composition | Structure |
|---|---|---|
| Vitamin D1 | Mixture of molecular compounds of ergocalciferol with lumisterol, 1:1 | |
| Vitamin D2 | ergocalciferol (made from ergosterol) | 
|
| Vitamin D3 | cholecalciferol
(made from 7-dehydrocholesterol in the skin).
|

|
| Vitamin D4 | 22-dihydroergocalciferol
|

|
| Vitamin D5 | sitocalciferol
(made from 7-dehydrositosterol )
|

|
Several forms (vitamers) of vitamin D exist.[1] The two major forms are vitamin D2 or ergocalciferol, and vitamin D3 or cholecalciferol.[1] Vitamin D without a subscript refers to either D2 or D3, or both, and is known collectively as calciferol.[citation needed]
Vitamin D2 was chemically characterized in 1931. In 1935, the chemical structure of vitamin D3 was defined and shown to result from the ultraviolet irradiation of 7-dehydrocholesterol. A chemical nomenclature for vitamin D forms was recommended in 1981,[16] but alternative names remain in common use.[4]
Chemically, the various forms of vitamin D are
Biology
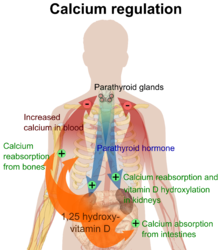
The active vitamin D metabolite calcitriol mediates its biological effects by binding to the vitamin D receptor (VDR), which is principally located in the nuclei of target cells.[1][17] The binding of calcitriol to the VDR allows the VDR to act as a transcription factor that modulates the gene expression of transport proteins (such as TRPV6 and calbindin), which are involved in calcium absorption in the intestine.[19] The vitamin D receptor belongs to the nuclear receptor superfamily of steroid/thyroid hormone receptors, and VDRs are expressed by cells in most organs, including the brain, heart, skin, gonads, prostate and breast.
VDR activation in the intestine, bone, kidney, and parathyroid gland cells leads to the maintenance of calcium and phosphorus levels in the blood (with the assistance of parathyroid hormone and calcitonin) and to the maintenance of bone content.[1][20]
One of the most important roles of vitamin D is to maintain skeletal calcium balance by promoting
The VDR regulates
Vitamin D receptor expression decreases with age.[1]
Deficiency
A diet with insufficient vitamin D in conjunction with inadequate sun exposure causes vitamin D deficiency, which is defined as a blood 25(OH)D level below 12 ng/mL (30 nmol/liter), whereas vitamin D insufficiency is a blood 25(OH)D level of 12–20 ng/mL (30–50 nmol/liter).[2][24] An estimated one billion adults worldwide are either vitamin D insufficient or deficient,[25] including in developed countries in Europe.[26] Severe vitamin D deficiency in children, a rare disease in the developed world, causes a softening and weakening of growing bones, and a condition called rickets.[27]
Vitamin D deficiency is found worldwide in the elderly and remains common in children and adults.[28][29][25] Deficiency results in impaired bone mineralization and bone damage which leads to bone-softening diseases,[30] including rickets in children and osteomalacia in adults. Low blood calcifediol (25-hydroxy-vitamin D) can result from avoiding the sun.[31] Being deficient in Vitamin D can cause the absorption of dietary calcium to fall from the normal fraction (between 60 and 80 percent) to as little as 15 percent.[20]
Dark-skinned people living in temperate climates have been shown to have low vitamin D levels.[32][33][34] Dark-skinned people are less efficient at making vitamin D because melanin in the skin hinders vitamin D synthesis.[35] Vitamin D deficiency is common in Hispanic and African-Americans in the United States, with levels dropping significantly in the winter.[24] This is due to the levels of melanin in the skin, as it acts as a natural protectant from sun exposure.[24]
Bone health
Rickets
Rickets, a childhood disease, is characterized by impeded growth and soft, weak, deformed
Maternal vitamin D deficiency may cause overt bone disease from before birth and impairment of bone quality after birth.[39][40] Nutritional rickets exists in countries with intense year-round sunlight such as Nigeria and can occur without vitamin D deficiency.[41][42]
Although rickets and osteomalacia are now rare in the United Kingdom, outbreaks have happened in some immigrant communities in which people with osteomalacia included women with seemingly adequate daylight outdoor exposure wearing Western clothing.[43] Having darker skin and reduced exposure to sunshine did not produce rickets unless the diet deviated from a Western omnivore pattern characterized by high intakes of meat, fish, and eggs.[44][45][46] The dietary risk factors for rickets include abstaining from animal foods.[43][47]
Vitamin D deficiency remains the main cause of rickets among young infants in most countries because breast milk is low in vitamin D and social customs and climatic conditions can prevent adequate sun exposure. In sunny countries such as Nigeria, South Africa, and Bangladesh, where rickets occurs among older toddlers and children, it has been attributed to low dietary calcium intakes, which are characteristic of cereal-based diets with limited access to dairy products.[46]
Rickets was formerly a major public health problem among the US population. In Denver, almost two-thirds of 500 children had mild rickets in the late 1920s.[48] An increase in the proportion of animal protein[47][49] in the 20th century American diet coupled with increased consumption of milk[50][51] fortified with relatively small quantities of vitamin D coincided with a dramatic decline in the number of rickets cases.[20] Also, in the United States and Canada, vitamin D-fortified milk, infant vitamin supplements, and vitamin supplements have helped to eradicate the majority of cases of rickets for children with fat malabsorption conditions.[30]
Osteomalacia and osteoporosis
Use of supplements
Supplementation with vitamin D is a reliable method for preventing or treating
A US
Mortality, all-causes
Vitamin D3 supplementation has been tentatively found to lead to a reduced risk of death in the elderly,[12][57] but the effect has not been deemed pronounced, or certain enough, to make taking supplements recommendable.[14] Other forms (vitamin D2, alfacalcidol, and calcitriol) do not appear to have any beneficial effects with regard to the risk of death.[12] High blood levels appear to be associated with a lower risk of death, but it is unclear if supplementation can result in this benefit.[61] Both an excess and a deficiency in vitamin D appear to cause abnormal functioning and premature aging.[62][63][64] The relationship between serum calcifediol concentrations and all-cause mortality is "U-shaped": mortality is elevated at high and low calcifediol levels, relative to moderate levels.[59] Harm from vitamin D appears to occur at a lower vitamin D level in the black population than in the white population.[59]: 435
Bone health
In general, no good evidence supports the commonly held belief that vitamin D supplements can help prevent
Athletes who are vitamin D deficient are at an increased risk of stress fractures and/or major breaks, particularly those engaging in contact sports. The greatest benefit with supplementation is seen in athletes who are deficient (25(OH)D serum levels <30 ng/mL), or severely deficient (25(OH)D serum levels <25 ng/mL). Incremental decreases in risks are observed with rising serum 25(OH)D concentrations plateauing at 50 ng/mL with no additional benefits seen in levels beyond this point.[71]
A 2020 Cochrane systematic review has found limited evidence that vitamin D plus calcium, but not independently can improve healing in children with nutritional rickets, but the evidence was not conclusive for reducing fractures.[72]
The US Food and Drug Administration (FDA) has required manufacturers to declare the amount of vitamin D on nutrition facts labels, as "nutrients of public health significance", since May 2016. By a proposed deadline extension, some manufacturers had until 1 July 2021, to comply.[73]
Cancer
Potential associations have been found between low vitamin D levels and the risk of developing several types of cancer.[74][75] Meta-analyses of observational studies have found reduced risk of cancer incidence related to vitamin D intake and 25(OH)D levels, particularly for colorectal cancer, although the strength of the associations was classified as weak.[75][76] Vitamin D receptor and SNAI2 are found to be involved in the metastastic process of osteosarcoma.[77] While randomized controlled trials have not confirmed that vitamin D supplements reduce the risk of cancer incidence, the relative risk of cancer deaths was lower by up to 16% in several meta-analyses.[78][76]
Cardiovascular disease
Vitamin D supplementation is not associated with a reduced risk of stroke, cerebrovascular disease, myocardial infarction, or ischemic heart disease.[14][79][80] Supplementation does not lower blood pressure in the general population.[81][82][83]
Immune system
Infectious diseases
In general, vitamin D functions to activate the innate and dampen the adaptive immune systems with antibacterial, antiviral and anti-inflammatory effects.[84][85] Low levels of vitamin D appear to be a risk factor for tuberculosis,[86] and historically it was used as a treatment.[87]
Vitamin D supplementation in low doses (400 to 1000 IU/day) may slightly decrease the overall risk of acute
Asthma
Vitamin D supplementation does not help prevent asthma attacks or alleviate their symptoms.[90]
Inflammatory bowel disease
Low levels of vitamin D are associated with two major forms of human inflammatory bowel disease: Crohn's disease and ulcerative colitis.[91] Deficiencies in vitamin D have been linked to the severity of the case of inflammatory bowel disease, however, whether vitamin D deficiency causes inflammatory bowel disease or is a symptom of the disease is not clear.[92]
There is some evidence that vitamin D supplementation therapy for people with inflammatory bowel disease may be associated with improvements in scores for clinical inflammatory bowel disease activity and biochemical markers.[93][92] Vitamin D treatment may be associated with less frequent relapse of symptoms in IBD.[92] It is not clear if this treatment improves the person's quality of life or what the clinical response to vitamin D treatment.[92] The ideal treatment regime and dose of vitamin D therapy has not been well enough studied.[92]
Other conditions
Diabetes
A meta-analysis reported that vitamin D supplementation significantly reduced the risk of type 2 diabetes for non-obese people with prediabetes.[94] Another meta-analysis reported that vitamin D supplementation significantly improved glycemic control [homeostatic model assessment-insulin resistance (HOMA-IR)], hemoglobin A1C (HbA1C), and fasting blood glucose (FBG) in individuals with type 2 diabetes.[95] In prospective studies, high versus low level of vitamin D was respectively associated with significant decrease in risk of type 2 diabetes, combined type 2 diabetes and prediabetes, and prediabetes.[96] A 2011 Cochrane systematic review examined one study that showed vitamin D together with insulin maintained levels of fasting C-peptide after 12 months better than insulin alone. However, it is important to highlight that the studies available to be included in this review presented considerable flaws in quality and design.[97]
Attention deficit hyperactivity disorder (ADHD)
A meta-analysis of observational studies showed that children with ADHD have lower vitamin D levels, and that there was a small association between low vitamin D levels at the time of birth and later development of ADHD.[98] Several small, randomized controlled trials of vitamin D supplementation indicated improved ADHD symptoms such as impulsivity and hyperactivity.[99]
Depression
Clinical trials of vitamin D supplementation for depressive symptoms have generally been of low quality and show no overall effect, although subgroup analysis showed supplementation for participants with clinically significant depressive symptoms or depressive disorder had a moderate effect.[100]
Cognition and dementia
A systematic review of clinical studies found an association between low vitamin D levels with
Schizophrenia
Trials have demonstrated lower vitamin D levels are highly prevalent in people with schizophrenia, particularly those with acute episodes.[102]
Pregnancy
Low levels of vitamin D in pregnancy are associated with gestational diabetes, pre-eclampsia, and small (for gestational age) infants.[103] Although taking vitamin D supplements during pregnancy raises blood levels of vitamin D in the mother at term,[104] the full extent of benefits for the mother or baby is unclear.[103][104][105] Pregnant women who take an adequate amount of vitamin D during gestation may experience a lower risk of pre-eclampsia[106] and positive immune effects.[107] Vitamin D supplementation is also likely to reduce the risk of gestational diabetes, undersized babies[106] and of their poor rate of growth.[108] Pregnant women often do not take the recommended amount of vitamin D.[107]
Weight loss
Though hypothesized that vitamin D supplementation may be an effective treatment for obesity apart from calorie restriction, one systematic review found no association of supplementation with body weight or fat mass.[109] A 2016 meta-analysis found that circulating vitamin D status was improved by weight loss, indicating that fat mass may be inversely associated with blood levels of vitamin D.[110]
Allowable health claims
Governmental regulatory agencies stipulate for the food and dietary supplement industries certain health claims as allowable as statements on packaging.
European Food Safety Authority
- normal function of the immune system[111]
- normal inflammatory response[111]
- normal muscle function[111]
- reduced risk of falling in people over age 60[112]
US Food and Drug Administration (FDA)
- "Adequate calcium and vitamin D, as part of a well balanced diet, along with physical activity, may reduce the risk of osteoporosis."[113]
- "Adequate calcium and regular exercise may help to achieve strong bones in children and adolescents and may reduce the risk of osteoporosis in older adults. An adequate intake of vitamin D is also necessary."[114]
Other possible agencies with claim guidance: Japan FOSHU[115] and Australia-New Zealand.[116]
Dietary intake
| United Kingdom | ||
| Age group | Intake (μg/day) | Maximum intake (μg/day)[117] |
|---|---|---|
| Breast-fed infants 0–12 months | 8.5 – 10 | 25 |
| Formula-fed infants (<500 mL/d) | 10 | 25 |
| Children 1 – 10 years | 10 | 50 |
| Children >10 and adults | 10 | 100 |
| United States | ||
| Age group | RDA (IU/day) | (μg/day)[59] |
| Infants 0–6 months | 400* | 10 |
| Infants 6–12 months | 400* | 10 |
| 1–70 years | 600 | 15 |
| Adults > 70 years | 800 | 20 |
| Pregnant/Lactating | 600 | 15 |
| Age group | Tolerable upper intake level (IU/day) | (μg/day) |
| Infants 0–6 months | 1,000 | 25 |
| Infants 6–12 months | 1,500 | 37.5 |
| 1–3 years | 2,500 | 62.5 |
| 4–8 years | 3,000 | 75 |
| 9+ years | 4,000 | 100 |
| Pregnant/lactating | 4,000 | 100[59] |
| Canada | ||
| Age group | RDA (IU)[118] | Tolerable upper intake (IU)[118] |
| Infants 0–6 months | 400* | 1,000 |
| Infants 7–12 months | 400* | 1,500 |
| Children 1–3 years | 600 | 2,500 |
| Children 4–8 years | 600 | 3,000 |
| Children and adults 9–70 years | 600 | 4,000 |
| Adults > 70 years | 800 | 4,000 |
| Pregnancy & lactation | 600 | 4,000 |
| Australia and New Zealand | ||
| Age group | Adequate Intake (μg)[116] | Upper Level of Intake (μg)[116] |
| Infants 0–12 months | 5* | 25 |
| Children 1–18 years | 5* | 80 |
| Adults 19–50 years | 5* | 80 |
| Adults 51–70 years | 10* | 80 |
| Adults > 70 years | 15* | 80 |
| European Food Safety Authority | ||
| Age group | Adequate Intake (μg)[119] | Tolerable upper limit (μg)[120] |
| Infants 0–12 months | 10 | 25 |
| Children 1–10 years | 15 | 50 |
| Children 11–17 years | 15 | 100 |
| Adults | 15 | 100 |
| Pregnancy & Lactation | 15 | 100 |
| * Adequate intake, no RDA/RDI yet established | ||
Recommended levels
Various institutions have proposed different recommendations for the amount of daily intake[121] of vitamin D. These vary according to precise definition, age, pregnancy or lactation, and the extent assumptions are made regarding skin synthesis of vitamin D.[117][59][118][116][119] Conversion: 1 μg (microgram) = 40 IU (international unit).[117]
United Kingdom
The UK National Health Service (NHS) recommends that people at risk of vitamin D deficiency, breast-fed babies, formula-fed babies taking less than 500 ml/day, and children aged 6 months to 4 years, should take daily vitamin D supplements throughout the year to ensure sufficient intake.[117] This includes people with limited skin synthesis of vitamin D, who are not often outdoors, are frail, housebound, living in a care home, or usually wearing clothes that cover up most of the skin, or with dark skin, such as having an African, African-Caribbean or south Asian background. Other people may be able to make adequate vitamin D from sunlight exposure from April to September. The NHS and Public Health England recommend that everyone, including those who are pregnant and breastfeeding, consider taking a daily supplement containing 10 μg (400 IU) of vitamin D during autumn and winter because of inadequate sunlight for vitamin D synthesis.[122]
United States
The
For US food and dietary supplement labeling purposes, the amount in a serving is expressed as a percent of Daily Value (%DV). For vitamin D labeling purposes, 100% of the daily value was 400 IU (10 μg), but in May 2016, it was revised to 800 IU (20 μg) to bring it into agreement with the recommended dietary allowance (RDA).[124][125] Compliance with the updated labeling regulations was required by 1 January 2020 for manufacturers with US$10 million or more in annual food sales, and by 1 January 2021 for manufacturers with lower volume food sales.[73][126] A table of the old and new adult daily values is provided at Reference Daily Intake.
Canada
Health Canada published recommended dietary intakes (DRIs) and tolerable upper intake levels (ULs) for vitamin D based on the jointly commissioned and funded Institute of Medicine 2010 report.[59][118]
Australia and New Zealand
Australia and New Zealand published nutrient reference values including guidelines for dietary vitamin D intake in 2006.[116] About a third of Australians have vitamin D deficiency.[127][128]
European Union
The European Food Safety Authority (EFSA) in 2016[119] reviewed the current evidence, finding the relationship between serum 25(OH)D concentration and musculoskeletal health outcomes is widely variable. They considered that average requirements and population reference intakes values for vitamin D cannot be derived, and that a serum 25(OH)D concentration of 50 nmol/L was a suitable target value. For all people over the age of 1, including women who are pregnant or lactating, they set an adequate intake of 15 μg/day (600 IU).[119]
The EFSA reviewed safe levels of intake in 2012,[120] setting the tolerable upper limit for adults at 100 μg/day (4000 IU), a similar conclusion as the IOM.
The Swedish National Food Agency recommends a daily intake of 10 μg (400 IU) of vitamin D3 for children and adults up to 75 years, and 20 μg (800 IU) for adults 75 and older.[129]
Non-government organisations in Europe have made their own recommendations. The German Society for Nutrition recommends 20 μg.[130] The European Menopause and Andropause Society recommends postmenopausal women consume 15 μg (600 IU) until age 70, and 20 μg (800 IU) from age 71. This dose should be increased to 100 μg (4,000 IU) in some patients with very low vitamin D status or in case of co-morbid conditions.[131]
Sources
Although vitamin D is present naturally in only a few foods,[2] it is commonly added as a fortification in manufactured foods. In some countries, staple foods are artificially fortified with vitamin D.[132]
Natural sources
| Animal sources | |||
| Source[133] | IU/g | Irregular | |
|---|---|---|---|
| Cooked egg yolk
|
0.7 | 44 IU for a 61g egg | |
| Beef liver, cooked, braised | 0.5 | ||
| Fish liver oils, such as cod liver oil | 100 | 450 IU per teaspoon (4.5 g) | |
| Fatty fish species | |||
| Salmon, pink, cooked, dry heat | 5.2 | ||
| Mackerel, Pacific and jack, mixed species, cooked, dry heat | 4.6 | ||
| Tuna, canned in oil | 2.7 | ||
Sardines, canned in oil , drained
|
1.9 | ||
| Fungal sources | |||
| Source | μg/g | IU/g | |
|---|---|---|---|
| Cladonia arbuscula (lichen), thalli, dry[134] | vitamin D3 | 0.67–2.04 | 27–82 |
| vitamin D2 | 0.22–0.55 | 8.8–22 | |
| Agaricus bisporus (common mushroom): D2 + D3 | |||
| Portobello | Raw | 0.003 | 0.1 |
| Exposed to ultraviolet light | 0.11 | 4.46 | |
| Crimini | Raw | 0.001 | 0.03 |
| Exposed to ultraviolet light | 0.32 | 12.8 | |
In general, vitamin D3 is found in animal source foods, particularly fish, meat, offal, egg and dairy.[135] Vitamin D2 is found in fungi and is produced by ultraviolet irradiation of ergosterol.[136] The vitamin D2 content in mushrooms and Cladina arbuscula, a lichen, increases with exposure to ultraviolet light,[134][137] and is stimulated by industrial ultraviolet lamps for fortification.[136] The United States Department of Agriculture reports D2 and D3 content combined in one value.
Food fortification
Manufactured foods fortified with vitamin D include some fruit juices and fruit juice drinks, meal replacement energy bars, soy protein-based beverages, certain cheese and cheese products, flour products, infant formulas, many breakfast cereals, and milk.[138][139]
In 2016 in the United States, the Food and Drug Administration (FDA) amended food additive regulations for milk fortification,[140] stating that vitamin D3 levels not exceed 42 IU vitamin D per 100 g (400 IU per US quart) of dairy milk, 84 IU of vitamin D2 per 100 g (800 IU per quart) of plant milks, and 89 IU per 100 g (800 IU per quart) in plant-based yogurts or in soy beverage products.[141][142][143] Plant milks are defined as beverages made from soy, almond, rice, among other plant sources intended as alternatives to dairy milk.[144]
While some studies have found that vitamin D3 raises 25(OH)D blood levels faster and remains active in the body longer,[145][146] others contend that vitamin D2 sources are equally bioavailable and effective as D3 for raising and sustaining 25(OH)D.[136][147][148]
Food preparation
Vitamin D content in typical foods is reduced variably by cooking. Boiled, fried and baked foods retained 69–89% of original vitamin D.[149]
Recommended serum levels

Recommendations on recommended 25(OH)D serum levels vary across authorities, and vary based on factors like age.[2] US labs generally report 25(OH)D levels in ng/mL.[152] Other countries often use nmol/L.[152] One ng/mL is approximately equal to 2.5 nmol/L.[153]
A 2014 review concluded that the most advantageous serum levels for 25(OH)D for all outcomes appeared to be close to 30 ng/mL (75 nmol/L).[154] The optimal vitamin D levels are still controversial and another review concluded that ranges from 30 to 40 ng/mL (75 to 100 nmol/L) were to be recommended for athletes.[155] Part of the controversy is because numerous studies have found differences in serum levels of 25(OH)D between ethnic groups; studies point to genetic as well as environmental reasons behind these variations.[156] Supplementation to achieve these standard levels could cause harmful vascular calcification.[34]
A 2012
In 2011 an
Excess
Vitamin D toxicity is rare.[25] It is caused by supplementing with high doses of vitamin D rather than sunlight. The threshold for vitamin D toxicity has not been established; however, according to some research:
- 100 μg/day (4
- 240 μg/day (10k IU), over 5 months have been show not to cause toxicity.[25]
- 1250 μg/day (50k IU) over several months can increase serum 25-hydroxyvitamin D levels to 150 ng/mL.[25][159]
Those with certain medical conditions, such as primary
Idiopathic infantile hypercalcemia is caused by a mutation of the CYP24A1 gene, leading to a reduction in the degradation of vitamin D. Infants who have such a mutation have an increased sensitivity to vitamin D and in case of additional intake a risk of hypercalcaemia.[162][163] The disorder can continue into adulthood.[164]
A review published in 2015 noted that adverse effects have been reported only at 25(OH)D serum concentrations above 200 nmol/L.[155]
Published cases of toxicity involving hypercalcemia in which the vitamin D dose and the 25-hydroxy-vitamin D levels are known all involve an intake of ≥40,000 IU (1,000 μg) per day.[160]
Those who are pregnant or breastfeeding should consult a doctor before taking a vitamin D supplement. The FDA advised manufacturers of liquid vitamin D supplements that droppers accompanying these products should be clearly and accurately marked for 400
Calcitriol itself is auto-regulated in a negative feedback cycle, and is also affected by parathyroid hormone, fibroblast growth factor 23, cytokines, calcium, and phosphate.[166]
A study published in 2017 assessed the prevalence of high daily intake levels of supplemental vitamin D among adults ages 20+ in the United States, based on publicly available
- Over 18% of the population exceeds the
- Over 3% of the population exceeds the NIH daily tolerable upper intake level (
- The percentage of the population taking over 1000 IU/day, as well as the percentage taking over 4000 IU/day, have both increased since 1999, according to trend analysis.[167]
Effect of excess
Vitamin D overdose causes hypercalcemia, which is a strong indication of vitamin D toxicity – this can be noted with an increase in urination and thirst. If hypercalcemia is not treated, it results in excess deposits of calcium in soft tissues and organs such as the kidneys, liver, and heart, resulting in pain and organ damage.[25][30][169]
The main symptoms of vitamin D overdose are hypercalcemia including
Vitamin D toxicity is treated by discontinuing vitamin D supplementation and restricting calcium intake. Kidney damage may be irreversible. Exposure to sunlight for extended periods of time does not normally cause vitamin D toxicity. The concentrations of vitamin D precursors produced in the skin reach an equilibrium, and any further vitamin D produced is degraded.[160]
Biosynthesis
Synthesis of vitamin D in nature is dependent on the presence of UV radiation and subsequent activation in the liver and in the kidneys. Many animals synthesize vitamin D3 from
Interactive pathway
Click on icon in lower right corner to open.
Click on genes, proteins and metabolites below to link to respective articles. [§ 1]
- ^ The interactive pathway map can be edited at WikiPathways: "VitaminDSynthesis_WP1531".
Photochemistry


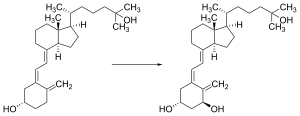
The transformation that converts 7-dehydrocholesterol to vitamin D3 occurs in two steps.
The conversion from ergosterol to vitamin D2 follows a similar procedure, forming previtamin D2 by photolysis, which isomerizes to vitamin D2 (ergocalciferol).[174] The transformation of previtamin D2 to vitamin D2 in methanol has a rate comparable to that of previtamin D3. The process is faster in white button mushrooms.[136]: fig. 3
Synthesis in the skin

Vitamin D3 is produced photochemically from 7-dehydrocholesterol in the skin of most vertebrate animals, including humans.
Adequate amounts of vitamin D can be produced with moderate sun exposure to the face, arms and legs (for those with the least melanin), averaging 5–30 minutes twice per week, or approximately 25% of the time for minimal sunburn. The darker the skin on the Fitzpatrick scale and the weaker the sunlight, the more minutes of exposure are needed. It also depends on parts of body exposed, all three factors affect minimal erythema dose (MED).[178] Vitamin D overdose from UV exposure is impossible: the skin reaches an equilibrium where the vitamin D degrades as fast as it is created.[25][179]
The skin consists of two primary layers: the inner layer called the
Evolution
Vitamin D can be synthesized only by a photochemical process. Its production from
Land vertebrates required another source of vitamin D other than plants for their calcified skeletons. They had to either ingest it or be exposed to sunlight to photosynthesize it in their skin.[170][173] Land vertebrates have been photosynthesizing vitamin D for more than 350 million years.[182]
In birds and fur-bearing mammals, fur or feathers block UV rays from reaching the skin. Instead, vitamin D is created from oily secretions of the skin deposited onto the feathers or fur, and is obtained orally during grooming.
Industrial synthesis
Vitamin D3 (cholecalciferol) is produced industrially by exposing
Mechanism of action
Metabolic activation

Vitamin D is carried via the blood to the liver, where it is converted into the prohormone calcifediol. Circulating calcifediol may then be converted into calcitriol – the biologically active form of vitamin D – in the kidneys.[190]
Whether synthesized in the skin or ingested, vitamin D is
Calcifediol is transported to the proximal tubules of the kidneys, where it is hydroxylated at the 1-α position (lower right of the molecule) to form calcitriol (1,25-dihydroxycholecalciferol, 1,25(OH)2D).
Inactivation
The activity of calcifediol and calcitriol can be reduced by hydroxylation at position 24 by vitamin D3 24-hydroxylase, forming secalciferol and calcitetrol, respectively.[4]
Difference between substrates
Vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol) share a similar mechanism of action as outlined above.[4] Metabolites produced by vitamin D2 are named with an er- or ergo- prefix to differentiate them from the D3-based counterparts (sometimes with a chole- prefix).[16]
- Metabolites produced from vitamin D2 tend to bind less well to the vitamin D-binding protein.[4]
- Vitamin D3 can alternatively be hydroxylated to calcifediol by sterol 27-hydroxylase (CYP27A1), but vitamin D2 cannot.[4]
- Ergocalciferol can be directly hydroxylated at position 24 by CYP27A1.[4] This hydroxylation also leads to a greater degree of inactivation: the activity of calcitriol decreases to 60% of original after 24-hydroxylation,[193] whereas ercalcitriol undergoes a 10-fold decrease in activity on conversion to ercalcitetrol.[194]
It is disputed whether these differences lead to a measurable drop in efficacy (see § Food fortification).
Intracellular mechanisms
Calcitriol enters the target cell and binds to the vitamin D receptor in the cytoplasm. This activated receptor enters the nucleus and binds to
Some reactions of the cell to calcitriol appear to be too fast for the classical VDRE transcription pathway, leading to the discovery of various non-genomic actions of vitamin D. The membrane-bound PDIA3 likely serves as an alternate receptor in this pathway.[195] The classical VDR may still play a role.[196]
History
Vitamin D was discovered in 1922 following on from previous research.[197] American researchers Elmer McCollum and Marguerite Davis in 1914[11] discovered a substance in cod liver oil which later was called "vitamin A". British doctor Edward Mellanby noticed dogs that were fed cod liver oil did not develop rickets and concluded vitamin A, or a closely associated factor, could prevent the disease. In 1922, Elmer McCollum tested modified cod liver oil in which the vitamin A had been destroyed.[11] The modified oil cured the sick dogs, so McCollum concluded the factor in cod liver oil which cured rickets was distinct from vitamin A. He called it vitamin D because he thought it was the fourth vitamin to be named.[198][199] It was not initially realized that vitamin D can be synthesized by humans (in the skin) through exposure to UV light, and therefore is technically not a vitamin, but rather can be considered to be a hormone.
In 1925,
In 1923, American biochemist
In 1969, a specific binding protein for vitamin D called the
Research
There is conflicting evidence about the benefits of interventions with vitamin D. Supplementation of between 800 and 1,000 IU is safe, but higher levels leading to blood levels of more than 50 ng/mL (125 nmol/L) may cause adverse effects.[2][210]
The US
Some preliminary studies link low vitamin D levels with disease later in life.[212] One meta-analysis found a decrease in mortality in elderly people.[12] Another meta-analysis covering over 350,000 people concluded that vitamin D supplementation in unselected community-dwelling individuals does not reduce skeletal (total fracture) or non-skeletal outcomes (myocardial infarction, ischemic heart disease, stroke, cerebrovascular disease, cancer) by more than 15%, and that further research trials with similar design are unlikely to change these conclusions.[14] As of 2022, there is insufficient evidence for an effect of vitamin D supplementation on the risk of cancer.[2][213][214] A 2019 meta-analysis found a small increase in risk of stroke when calcium and vitamin D supplements were taken together.[215]
COVID-19
As of September 2022[update] the US National Institutes of Health state there is insufficient evidence to recommend for or against using vitamin D supplementation to prevent or treat COVID-19.[216] The UK National Institute for Health and Care Excellence (NICE) does not recommend to offer a vitamin D supplement to people solely to prevent or treat COVID-19.[217][218] Both organizations included recommendations to continue the previous established recommendations on vitamin D supplementation for other reasons, such as bone and muscle health, as applicable. Both organizations noted that more people may require supplementation due to lower amounts of sun exposure during the pandemic.[216][217]
Several systematic reviews and meta-analyses of multiple studies have described the associations of vitamin D deficiency with adverse outcomes in COVID-19.[219][220][221][222][223][224] In the largest analysis, with data from 76 observational studies including almost two million adults, vitamin D deficiency or insufficiency significantly increased the susceptibility to becoming infected with COVID-19 and having severe COVID-19, with odds ratios of 1.5 and 1.9 respectively, but these findings had high risk of bias and heterogeneity. A two-fold greater mortality was found, but this analysis was less robust.[224] These findings confirm smaller, earlier analyses,[220][221][222][223] one of which, in reporting that people with COVID-19 tend to have lower 25(OH)D levels than healthy subjects, stated that the trend for associations with health outcomes was limited by the low quality of the studies and by the possibility of reverse causality mechanisms.[222]
A meta-analysis of three studies on the effect of oral vitamin D or calcifediol supplementation indicated a lower intensive care unit (ICU) admission rate (odds ratio: 0.36) compared to those without supplementation, but without a change in mortality.[225] A Cochrane review, also of three studies, found the evidence for the effectiveness of vitamin D supplementation for the treatment of COVID-19 to be very uncertain.[226] They found there was substantial clinical and methodological heterogeneity in the three studies that were included, mainly because of different supplementation strategies, vitamin D formulations (one using calcifediol), pre-treatment status and reported outcomes.[226] Another meta-analysis stated that the use of high doses of vitamin D in people with COVID-19 is not based on solid evidence although calcifediol supplementation may have a protective effect on ICU admissions.[222]
Other animals
Fish
Fish do not synthesise vitamin D in a natural setting and rely on dietary sources. As with mammals, vitamin D3 is more bioavailable than vitamin D2.[227] Unlike mammals, both hydroxylation steps from vitamin D3 to the active form 1,25 hydroxyvitamin D3 occur in the liver, so plasma levels of 25 hydroxyvitamin D3 is not an accurate measure of vitamin D3 levels.[227]
References
- ^ a b c d e f g h i j k l m n o p q r s t u v w x "Vitamin D". Micronutrient Information Center, Linus Pauling Institute, Oregon State University, Corvallis. 11 February 2021. Archived from the original on 8 April 2015. Retrieved 14 March 2022.
- ^ a b c d e f g h i j k l m n o p q "Vitamin D". Office of Dietary Supplements, US National Institutes of Health. 12 August 2022. Archived from the original on 9 April 2021. Retrieved 22 February 2022.
- ^ PMID 18689389.
- ^ PMID 24529992.
- PMID 26354531.
- ^ Cardwell, Glenn et al. “A Review of Mushrooms as a Potential Source of Dietary Vitamin D.” Nutrients vol. 10,10 1498. 13 Oct. 2018, doi:10.3390/nu10101498
- ^ "Vitamin D Tests". Lab Tests Online (USA). American Association for Clinical Chemistry. Archived from the original on 7 November 2017. Retrieved 23 June 2013.
- S2CID 35887181.
- ^ PMID 4323790.
- ^ S2CID 35236666.
- ^ PMID 15173387.
- ^ PMID 24414552.
- ^ S2CID 37968189.
- ^ PMID 24703049.
- ^ "The Lancet Diabetes & Endocrinology: Vitamin D supplementation in adults does not prevent fractures, falls or improve bone mineral density". EurekAlert!. Archived from the original on 24 March 2022. Retrieved 23 February 2022.
The authors conclude that there is therefore little reason to use vitamin D supplements to maintain or improve musculoskeletal health, except for the prevention of rare conditions such as rickets and osteomalacia in high risk groups, which can be caused by vitamin D deficiency after long lack of exposure to sunshine.
- ^ PMID 7094913.
- ^ ISBN 978-0-323-66162-1.
- ISBN 978-1-4557-3328-6. Archivedfrom the original on 19 March 2023. Retrieved 9 April 2017.
- S2CID 9853381.
- ^ PMID 15585788.
- ^ PMID 20506379.
- PMID 25741906.
- PMID 9011759.
- ^ PMID 21646368.
- ^ S2CID 18566028.
- PMID 26864360.
- ^ "Rickets". National Health Service. 8 March 2012. Archived from the original on 11 October 2017. Retrieved 9 July 2012.
- PMID 26745253.
- S2CID 22112344.
- ^ ISBN 978-1-285-82025-5. Archivedfrom the original on 19 March 2023. Retrieved 9 April 2017.
- PMID 18234141.
- PMID 28620422.
- PMID 24267433.
- ^ S2CID 29026212.
- PMID 28467404.
- ^ S2CID 342161.
- PMID 17943890.
- PMID 10824056.
- S2CID 14727399.
- PMID 25552383.
- S2CID 15406992.
- PMID 10584471.
- ^ PMID 9483661.
- PMID 6970590.
- .
- ^ PMID 15585795.
- ^ PMID 16351777.
- PMID 4862158.
- ISBN 978-0-87983-826-3. Archivedfrom the original on 19 March 2023. Retrieved 9 April 2017.
- ISBN 978-0-8147-1938-1. Archivedfrom the original on 19 March 2023. Retrieved 9 April 2017.
- PMID 10232644.
- PMID 16529140.
- S2CID 17244398.
- S2CID 30189971.
- PMID 20629479.
- PMID 24690624.
- ^ PMID 24622671.
- S2CID 23994681.
- ^ from the original on 26 January 2021. Retrieved 17 September 2017.
- ^ (PDF) from the original on 3 August 2020. Retrieved 17 November 2011.
- PMID 24938302.
- S2CID 40625040.
- S2CID 17462970.
- S2CID 36692930.
- PMID 24729336.
- (PDF) from the original on 15 December 2020. Retrieved 17 July 2019.
- S2CID 22380502.
- PMID 29279934.
- PMID 18088161.
- PMID 24768505.
- PMID 24179588.
- PMID 32303107.
- ^ a b "Changes to the Nutrition Facts Label". U.S. Food and Drug Administration (FDA). 27 May 2016. Archived from the original on 6 May 2018. Retrieved 16 May 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- PMID 26822497.
- ^ S2CID 235746547.
- ^ PMID 33553987.
- PMID 37054849.
- PMID 30796437.
- PMID 31215980.
- PMID 32462077.
- PMID 25775274.
- PMID 31922371.
- PMID 33618764.
- PMID 21419266.
- PMID 32904944.
- PMID 18245055.
- S2CID 18802134.
- ^ a b "SACN rapid review: Vitamin D and acute respiratory tract infections". Public Health England. Archived from the original on 14 January 2021. Retrieved 6 January 2021.
- S2CID 58548871.
- PMID 36744416.
- PMID 26348447.
- ^ PMID 37781953.
- PMID 32385487.
- S2CID 219897727.
- S2CID 57479957.
- S2CID 235295924.
- PMID 21901702.
- PMID 29438455.
- S2CID 199054851.
- PMID 24632894.
- PMID 23008220.
- PMID 25489478.
- ^ PMID 23533188.
- ^ PMID 26877200.
- PMID 29187358.
- ^ PMID 31348529.
- ^ PMID 22666547.
- PMID 29813153.
- S2CID 8660739.
- PMID 27604772.
- ^ .
- (PDF) from the original on 20 August 2019. Retrieved 20 August 2019.
- ^ "Guidance for Industry: Food Labeling Guide". Food and Drug Administration (FDA). January 2013. Archived from the original on 22 December 2020. Retrieved 17 July 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Health Canada Scientific Summary on the U. S. Health Claim Regarding Calcium and Osteoporosis". Bureau of Nutritional Sciences Food Directorate, Health Products and Food Branch Health Canada. 1 May 2000. Archived from the original on 3 December 2016. Retrieved 29 January 2012.
- ^ "Regulatory Systems of Health Claims in Japan" (PDF). Japan Consumer Affairs Agency, Food Labelling Division. 1 June 2011. Archived from the original (PDF) on 6 March 2012. Retrieved 29 January 2012.
- ^ ISBN 1-86496-243-7. Archivedfrom the original on 3 March 2023. Retrieved 19 March 2023.
- ^ a b c d "Vitamins and minerals – Vitamin D". National Health Service. 3 August 2020. Archived from the original on 30 October 2017. Retrieved 15 November 2020.
- ^ a b c d "Vitamin D and Calcium: Updated Dietary Reference Intakes". Nutrition and Healthy Eating. Health Canada. 5 December 2008. Archived from the original on 14 June 2017. Retrieved 28 April 2018.
- ^ hdl:11380/1228918.
- ^ hdl:2434/257871.
- ^ "Office of Dietary Supplements – Vitamin D". National Institutes of Health Office of Dietary Supplements. Archived from the original on 23 July 2020. Retrieved 14 April 2022.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "PHE publishes new advice on vitamin D". Public Health England. 21 July 2016. Archived from the original on 3 January 2021. Retrieved 15 November 2020.
- ^ "Vitamin D". The Nutrition Source. 18 September 2012. Archived from the original on 13 April 2022. Retrieved 14 April 2022.
- ^ "Federal Register May 27, 2016 Food Labeling: Revision of the Nutrition and Supplement Facts Labels. FR page 33982" (PDF). Archived (PDF) from the original on 8 August 2016. Retrieved 20 August 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Daily Value Reference of the Dietary Supplement Label Database (DSLD)". Dietary Supplement Label Database (DSLD). Archived from the original on 7 April 2020. Retrieved 16 May 2020.
- ^ "Industry Resources on the Changes to the Nutrition Facts Label". U.S. Food and Drug Administration (FDA). 21 December 2018. Archived from the original on 25 December 2020. Retrieved 16 May 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ Salleh A (12 June 2012). "Vitamin D food fortification on the table". Australian Broadcasting Corporation. Archived from the original on 22 December 2020. Retrieved 12 June 2012.
- ^ "Australian Health Survey: Biomedical Results for Nutrients, 2011–12". Australian Bureau of Statistics. 21 December 2011. Archived from the original on 10 March 2023. Retrieved 19 March 2023.
- ^ "Vitamin D (translated)" (in Swedish). Swedish National Food Agency. Archived from the original on 26 October 2020. Retrieved 19 October 2018.
- ^ Vitamin-D-Bedarf bei fehlender endogener Synthese Deutsche Gesellschaft für Ernährung, January 2012
- PMID 22100145.
- from the original on 2 April 2015. Retrieved 11 April 2010.
- ^ "Search, National Nutrient Database for Standard Reference Release 27". US Department of Agriculture, Agricultural Research Service. 2014. Archived from the original on 19 April 2014. Retrieved 12 June 2015.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ from the original on 28 May 2020. Retrieved 31 October 2018.
- PMID 23858093.
- ^ PMID 24494050.
- ^ Haytowitz DB (2009). "Vitamin D in mushrooms" (PDF). Nutrient Data Laboratory, US Department of Agriculture. Archived (PDF) from the original on 1 February 2021. Retrieved 16 April 2018.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- PMID 22481896.
- PMID 25635171.
- ^ "Vitamin D for Milk and Milk Alternatives". Food and Drug Administration (FDA). 15 July 2016. Archived from the original on 22 December 2020. Retrieved 22 February 2017.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Federal Register: Food Additives Permitted for Direct Addition to Food for Human Consumption; Vitamin D2". Food and Drug Administration, US Department of Health and Human Services. 18 July 2016. Archived from the original on 22 December 2020. Retrieved 22 February 2017.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "§172.379 Vitamin D2". Electronic Code of Federal Regulations. Archived from the original on 22 December 2020. Retrieved 16 July 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "§172.380 Vitamin D3". Electronic Code of Federal Regulations. Archived from the original on 22 December 2020. Retrieved 16 July 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Alternative to dairy milk". osoblanco. 16 January 2020. Archived from the original on 22 December 2020. Retrieved 20 January 2020.
- .
- PMID 24067388.
- PMID 23386645.
- (PDF) from the original on 13 July 2021. Retrieved 27 April 2021.
- PMID 24262542.
- from the original on 19 March 2023. Retrieved 20 August 2019.
- S2CID 5929230.
- ^ a b "25(OH)D levels in ng/mL". health harvard edu/. 19 December 2016. Archived from the original on 2 January 2020. Retrieved 2 January 2020.
- ^ "nmol converter". endmemo. Archived from the original on 2 February 2020. Retrieved 5 January 2020.
- PMID 25207384.
- ^ PMID 26288575.
- PMID 18593774.
- PMID 23149428.
- ^ PMID 21118827.
- ^ a b c Vitamin D at Merck Manual of Diagnosis and Therapy Professional Edition
- ^ (PDF) from the original on 3 July 2012. Retrieved 30 January 2011.
- ISBN 978-92-9199-014-6. Archived(PDF) from the original on 6 May 2019. Retrieved 27 February 2011.
- PMID 21675912.
- PMID 31188746.
- PMID 27588937.
- ^ "FDA Cautions on Accurate Vitamin D Supplementation for Infants" (Press release). Food and Drug Administration (FDA). 15 June 2010. Archived from the original on 12 January 2017.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- PMID 25584965.
- ^ PMID 28632857.
- from the original on 29 January 2016. Retrieved 7 July 2012.
- ^ ISBN 978-1-284-06465-0. Archivedfrom the original on 19 March 2023. Retrieved 9 April 2017.
- ^ PMID 1297827.
- PMID 3030826.
- PMID 24466410.
- ^ PMID 14985208.
- .
- PMID 12514284.
- ISBN 978-0-12-809965-0.
- S2CID 221636019.
108 references
- PMID 34580202.
- S2CID 87725403.
- S2CID 6072459.
- PMID 24466411.
- ISBN 978-1-101-22293-5. Archivedfrom the original on 19 March 2023. Retrieved 19 March 2023.
- ISBN 978-1-4419-8891-1. Archived(PDF) from the original on 29 January 2006.
The high 25(OH)D concentrations, and relatively high vitamin D requirements of apes and monkeys are understandable in light of their biology—their body surface area relative to mass is generally greater than for humans, and they are inveterate groomers, consuming by mouth the vitamin D generated from the oils secreted by skin into fur. Although much of the vitamin D produced within human skin is absorbed directly, birds and furbearing animals acquire most of their vitamin D orally, as they groom themselves (Bicknell and Prescott, 1946; Carpenter and Zhao, 1999). Vitamin D is generated from the oily secretions of skin into fur. The oral consumption of UV-exposed dermal excretion is the way many animals acquire the "nutrient," vitamin D. Although Fraser (1983) has argued that dermal absorption of vitamin D may be more natural, what we know from animals indicates that oral consumption is equally physiological. Since vitamin D can be extracted from UV-exposed human sweat and skin secretions (Bicknell and Prescott, 1946), it is also reasonable to think that early humans obtained some of their vitamin D by mouth as well, by licking the skin.
- PMID 8384476.
- PMID 31803981.
- ^ (PDF) from the original on 18 November 2017. Retrieved 24 November 2011.
[Vitamin D3] is produced commercially by extracting 7-dehydrocholesterol from wool fat, followed by UVB irradiation and purification [...] [Vitamin D2] is commercially made by irradiating and then purifying the ergosterol extracted from yeast
- from the original on 1 November 2018. Retrieved 20 August 2019.
- S2CID 53437216. Retrieved 2 December 2023.
- PMID 23717318.
- ^ PMID 20133466.
- PMID 15128933.
- ISBN 978-0-12-252687-9. Archivedfrom the original on 19 March 2023. Retrieved 9 April 2017.
- PMID 4355503.
- PMID 3013880.
- PMID 25448737.
- PMID 26950144.
- PMID 35245207.
- ^ Carere S (25 July 2007). "Age-old children's disease back in force". Toronto Star. Archived from the original on 17 May 2008. Retrieved 24 August 2010.
- ISBN 978-0-12-709821-0. Archivedfrom the original on 19 March 2023. Retrieved 19 March 2023.
- ^ "History of Vitamin D". University of California at Riverside. 2011. Archived from the original on 16 October 2017. Retrieved 9 May 2014.
- ^ "Adolf Windaus – Biography". Nobelprize.org. 25 March 2010. Archived from the original on 24 July 2018. Retrieved 25 March 2010.
- .
- JSTOR 81571.
- ISBN 978-0-12-387035-3. Archivedfrom the original on 19 March 2023. Retrieved 19 March 2023.
- ISBN 978-0-8047-4920-6. Archivedfrom the original on 19 March 2023. Retrieved 19 March 2023.
- ISBN 978-1-164-49678-6. Archivedfrom the original on 19 March 2023. Retrieved 9 April 2017.
- PMID 5253652.
- PMID 4332591.
- PMID 6251551.
- PMID 32857334.
- ^ "ODS Vitamin D Initiative". Office of Dietary Supplements, US National Institutes of Health. 2018. Archived from the original on 26 January 2021. Retrieved 22 February 2023.
- S2CID 13296456.
- ^ "Vitamin D and cancer prevention". National Cancer Institute, US National Institutes of Health. 21 October 2013. Archived from the original on 13 February 2015. Retrieved 15 December 2016.
- PMID 29635490.
- PMID 31284304.
- ^ a b "Vitamin D". Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health (NIH). 26 September 2022. Retrieved 4 July 2023.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ ISBN 978-1-4731-3942-8. NG187. Archivedfrom the original on 3 December 2021. Retrieved 22 February 2021.
- ^ Evidence reviews for the use of vitamin D supplementation as prevention and treatment of COVID-19 (PDF) (Report). National Institute for Health and Care Excellence (NICE). December 2020. Archived from the original on 20 October 2021. Retrieved 22 February 2021.
- PMID 33401034.
- ^ PMID 33751020.
- ^ PMID 33775818.
- ^ PMID 33774074.
- ^ S2CID 236997712.
- ^ PMID 34894254.
- PMID 33486522.
- ^ S2CID 235202971.
- ^ ISSN 1753-5123.