Organopalladium chemistry
Organopalladium chemistry is a branch of
Organopalladium chemistry timeline
- 1873 - A. N. Zaitsev reports reduction of benzophenoneover palladium with hydrogen.
- 1894 - Phillips reports that
- 1907 - Autoclave technology introduced by Vladimir Ipatieff makes it possible to carry out high pressure hydrogenation.
- 1956 - In the Wacker process ethylene and oxygen react to acetaldehyde with catalyst PdCl2/CuCl2
- 1957 - Tetrakis(triphenylphosphine)palladium(0) is reported by Malatesta and Angoletta.
- 1972 - The Heck reaction is a coupling reaction of a halogenide with an olefin. Pd(0) intermediates are implicated.
- 1973 - The Trost asymmetric allylic alkylation is a nucleophilic substitution.
- 1975 - The Sonogashira coupling is a coupling reaction of terminal alkynes with aryl or vinyl halides.
- 1994 - The Pd-catalyzed Buchwald-Hartwig aminationfor C-N bond-forming reactions.
Palladium(II)
Alkene complexes
Unlike Ni(II), but similar to Pt(II), Pd(II) halides form a variety of alkene complexes. The premier example is

Palladium(II) acetate and related compounds are common reagents because the carboxylates are good leaving groups with basic properties. For example palladium trifluoroacetate has been demonstrated to be effective in aromatic decarboxylation:[3]
Allyl complexes
The iconic complex in this series is
Allylpalladium intermediates also feature in the
Palladium-carbon sigma-bonded complexes
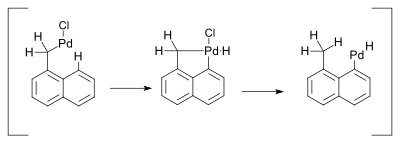
Various organic groups can bound to palladium and form stable sigma-bonded complexes. The stability of the bonds in terms of bond dissociation energy follows the trend: Pd-Alkynyl > Pd-Vinyl ≈ Pd-Aryl > Pd-Alkyl and the metal-carbon bond length changes in the opposite direction: Pd-Alkynyl < Pd-Vinyl ≈ Pd-Aryl < Pd-Alkyl.[7]
Palladium(0) compounds
Organopalladium(IV)
The first organopalladium(IV) compound was described in 1986. This complex is Me3Pd(IV)(I)bpy (bpy = bidentate
Palladium compounds owe their reactivity to the ease of interconversion between Pd(0) and palladium(II) intermediates. There is no conclusive evidence however for the involvement of Pd(II) to Pd(IV) conversions in palladium mediated organometallic reactions.[9] One reaction invoking such mechanism was described in 2000 and concerned a Heck reaction. This reaction was accompanied by a 1,5-hydrogen shift in the presence of amines:[10]
The hydride shift was envisaged as taking place through a Pd(IV) metallacycle:
In related work the intermediate associated with the hydride shift remains Pd(II):[11]
and in other work (a novel synthesis of indoles with two Pd migrations) equilibria are postulated between different palladacycles:[12][13]
and in certain intramolecular couplings synthetic value was demonstrated regardless of oxidation state:[14]
See also
References
- ISBN 0-471-31506-0
- ^ Phillips, F. C.; Am. Chem. J. 1894, 16, 255.
- PMID 17542594.
- ^ Jan-E. Bäckvall and Jan O. Vågberg (1993). "Stereoselective 1,4-Functionalizations of Conjugated Dienes: cis- and trans-1-Acetoxy-4-(Dicarbomethoxymethyl)-2-Cyclohexene". Organic Syntheses; Collected Volumes, vol. 8, p. 5.
- PMID 17960935.
- DBU (is reported to absorb the amine protons that would otherwise trigger isomerization) in THF
- .
- PMID 18001098.
- .
- (CsPiv)