Organosulfur chemistry
Organosulfur chemistry is the study of the properties and synthesis of organosulfur compounds, which are
Sulfur shares the chalcogen group with oxygen, selenium, and tellurium, and it is expected that organosulfur compounds have similarities with carbon–oxygen, carbon–selenium, and carbon–tellurium compounds.
A classical chemical test for the detection of sulfur compounds is the Carius halogen method.
Structural classes
Organosulfur compounds can be classified according to the sulfur-containing functional groups, which are listed (approximately) in decreasing order of their occurrence.
- Illustrative organosulfur compounds
-
Allicin, the active flavor compound in crushed garlic
-
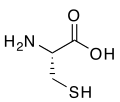
(R)-Cysteine, an amino acid containing a thiol group
-
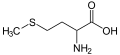
Methionine, an amino acid containing a sulfide
-
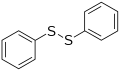
Diphenyl disulfide, a representative disulfide
-
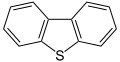
Dibenzothiophene, a component of crude oil
-
Perfluorooctanesulfonic acid, a controversial surfactant
-
Lipoic acid, an essential cofactor of four mitochondrial enzyme complexes.
-
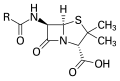
Penicillin core structure, where "R" is the variable group.
-
sulfa drug.
-
chemical warfare agent.
-
Martin's sulfurane with a see-saw structure, like that of SF4[2]
Sulfides
Sulfides, formerly known as thioethers, are characterized by C−S−C
Sulfides are typically prepared by
They can also be prepared via the Pummerer rearrangement.
In the
Thioacetals and thioketals feature C−S−C−S−C bond sequence. They represent a subclass of sulfides. The thioacetals are useful in "umpolung" of carbonyl groups. Thioacetals and thioketals can also be used to protect a carbonyl group in organic syntheses.
The above classes of sulfur compounds also exist in saturated and unsaturated
Thiols, disulfides, polysulfides
The difference in
Certain aromatic thiols can be accessed through a Herz reaction.
Longer sulfur chains are also known, such as in the natural product varacin which contains an unusual pentathiepin ring (5-sulfur chain cyclised onto a benzene ring).
Thioesters
Thioesters have general structure R−C(O)−S−R. They are related to regular esters (R−C(O)−O−R) but are more susceptible to hydrolysis and related reactions. Thioesters formed from coenzyme A are prominent in biochemistry, especially in fatty acid synthesis.
Sulfoxides, sulfones and thiosulfinates
A
Sulfimides, sulfoximides, sulfonediimines
Sulfimides (also called a sulfilimines) are sulfur–nitrogen compounds of structure R2S=NR′, the nitrogen analog of sulfoxides. They are of interest in part due to their pharmacological properties. When two different R groups are attached to sulfur, sulfimides are chiral. Sulfimides form stable α-carbanions.[9]
Sulfoximides (also called sulfoximines) are tetracoordinate sulfur–nitrogen compounds, isoelectronic with sulfones, in which one oxygen atom of the sulfone is replaced by a substituted nitrogen atom, e.g., R2S(O)=NR′. When two different R groups are attached to sulfur, sulfoximides are chiral. Much of the interest in this class of compounds is derived from the discovery that methionine sulfoximide (methionine sulfoximine) is an inhibitor of glutamine synthetase.[10]
Sulfonediimines (also called sulfodiimines, sulfodiimides or sulfonediimides) are tetracoordinate sulfur–nitrogen compounds, isoelectronic with sulfones, in which both oxygen atoms of the sulfone are replaced by a substituted nitrogen atom, e.g., R2S(=NR′)2. They are of interest because of their biological activity and as building blocks for heterocycle synthesis.[11]
S-Nitrosothiols
S-Nitrosothiols, also known as thionitrites, are compounds containing a nitroso group attached to the sulfur atom of a thiol, e.g. R−S−N=O. They have received considerable attention in biochemistry because they serve as donors of the nitrosonium ion, NO+, and nitric oxide, NO, which may serve as signaling molecules in living systems, especially related to vasodilation.[12]
Sulfur halides
A wide range of organosulfur compounds are known which contain one or more
Compounds with
S-Oxides and S,S-dioxides of thiocarbonyl compounds
The S-oxides of thiocarbonyl compounds are known as thiocarbonyl S-oxides: (R2C=S=O, and thiocarbonyl S,S-dioxides or
Triple bonds between carbon and sulfur
Triple bonds between sulfur and carbon in sulfaalkynes are rare and can be found in carbon monosulfide (CS) [20] and have been suggested for the compounds F3CCSF3[21][22] and F5SCSF3.[23] The compound HCSOH is also represented as having a formal triple bond.[24]
Thiocarboxylic acids and thioamides
Thiocarboxylic acids (RC(O)SH) and dithiocarboxylic acids (RC(S)SH) are well known. They are structurally similar to carboxylic acids but more acidic. Thioamides are analogous to amides.
A
Sulfonium, oxosulfonium and thiocarbonyl ylides
Deprotonation of sulfonium and oxosulfonium salts affords
Sulfuranes and persulfuranes
Sulfuranes are relatively specialized functional group that feature
One of the few all-carbon persulfuranes has two
It is prepared from the corresponding sulfurane 1 with
Organosulfur compounds in nature
A variety or organosulfur compounds occur in nature. Most abundant are the amino acids methionine, cysteine, and cystine. The vitamins biotin and thiamine, as well as lipoic acid contain sulfur heterocycles. Glutathione is the primary intracellular antioxidant.[6] Penicillin and cephalosporin are life-saving antibiotics, derived from fungi. Gliotoxin is a sulfur-containing mycotoxin produced by several species of fungi under investigation as an antiviral agent.
In fossil fuels
Common organosulfur compounds present in petroleum fractions at the level of 200–500 ppm. Common compounds are thiophenes, especially dibenzothiophenes. By the process of hydrodesulfurization (HDS) in refineries, these compounds are removed as illustrated by the hydrogenolysis of thiophene: C4H4S + 8 H2 → C4H10 + H2S
Flavor and odor
Compounds like
Humans and other animals have an exquisitely sensitive sense of smell toward the odor of low-valent organosulfur compounds such as thiols, sulfides, and disulfides. Malodorous volatile thiols are protein-degradation products found in putrid food, so sensitive identification of these compounds is crucial to avoiding intoxication. Low-valent volatile sulfur compounds are also found in areas where oxygen levels in the air are low, posing a risk of suffocation.
Copper is required for the highly sensitive detection of certain volatile thiols and related organosulfur compounds by olfactory receptors in mice. Whether humans, too, require copper for sensitive detection of thiols is not yet known.[31]
References
- ISBN 0-12-107050-6.
- ^ .
- ^ Organic chemistry IUPAC Blue Book. Rules C-5: Compounds Containing Bivalent Sulfur http://www.acdlabs.com/iupac/nomenclature/79/r79_25.htm
- ^ Organic chemistry IUPAC Blue Book. Recommendation R-5.7.1.3.4 Thiocarboxylic and thiocarbonic acids.[1]
- ISBN 0-8493-0481-4.
- ^ PMID 25144663.
- .
- ISBN 0-471-95512-4.
- ISBN 978-1-58890-530-7.
- ISBN 978-1-58890-530-7.
- ISBN 978-1-58890-530-7.
- PMID 15749378.
- ISBN 978-1-58890-530-7.
- .
- ISBN 978-1-58890-530-7.
- .
- .
- .
- .
- .
- .
- .
- PMID 19768827.
- ^ Organic chemistry IUPAC Blue Book. C-6 Sulfur Halides, Sulfoxides, Sulfones, and Sulfur Acids and Their Derivatives http://www.acdlabs.com/iupac/nomenclature/79/r79_26.htm
- ISBN 978-1-58890-530-7.
- ISBN 978-1-58890-530-7.
- .
- PMID 16719444.
- ISBN 978-0-8412-2616-6.
- PMID 22328155.



![Martin's sulfurane with a see-saw structure, like that of SF4[2]](http://upload.wikimedia.org/wikipedia/commons/thumb/3/36/MartinSulfurane.svg/96px-MartinSulfurane.svg.png)

