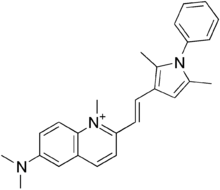
Pyrvinium
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| ATC code | |
| Identifiers | |
| |
JSmol) | |
| |
| |
Pyrvinium (Viprynium) is an
pamoate.[2][3] Pyrvinium was identified as a potent Wnt inhibitor, acting through activation of Casein kinase CK1α.[4][5]
Pyrvinium salts can also inhibit the growth of cancer cells.[6] More specifically, the pamoate salt has been shown to have preferential toxicity for various cancer cell lines during glucose starvation.[7]
Synthesis
One synthetic method is based on
Paal-Knorr synthesis.[6] More recently, an alternative convergent, synthetic strategy to pyrvinium triflate salts through Friedländer synthesis was reported.[3]
References
- PMID 14027194.
- ^ "Pyrvinium". PubChem. U.S. National Library of Medicine.
- ^ doi:10.3987/COM-12-12446 (inactive 2024-02-17).)
{{cite journal}}: CS1 maint: DOI inactive as of February 2024 (link - PMID 21170416.
- PMID 32824859.
- ^ a b WO 2006078754, Macdonald JE, Hysell MK, Yu D, Li H, Wong-Staal F, "Novel Quinolinium Salts and Derivatives", published 2006-07-27
- PMID 15298733.
