Phagocytosis


Phagocytosis (from

In a multicellular organism's immune system, phagocytosis is a major mechanism used to remove pathogens and cell debris. The ingested material is then digested in the phagosome. Bacteria, dead tissue cells, and small mineral particles are all examples of objects that may be phagocytized. Some protozoa use phagocytosis as means to obtain nutrients.
History
The
The first demonstration of phagocytosis as a property of leucocytes, the immune cells, was from the German zoologist
Phagocytosis was noted by Canadian physician William Osler (1876),[9] and later studied and named by Élie Metchnikoff (1880, 1883).[10]
In immune system
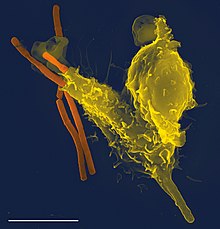
Phagocytosis is one main mechanisms of the innate immune defense. It is one of the first processes responding to infection, and is also one of the initiating branches of an adaptive immune response. Although most cells are capable of phagocytosis, some cell types perform it as part of their main function. These are called 'professional phagocytes.' Phagocytosis is old in evolutionary terms, being present even in invertebrates.[11]
Professional phagocytic cells
Neutrophils, macrophages, monocytes, dendritic cells, osteoclasts and eosinophils can be classified as professional phagocytes.[10] The first three have the greatest role in immune response to most infections.[11]
The role of neutrophils is patrolling the bloodstream and rapid migration to the tissues in large numbers only in case of infection.[11] There they have direct microbicidal effect by phagocytosis. After ingestion, neutrophils are efficient in intracellular killing of pathogens. Neutrophils phagocytose mainly via the Fcγ receptors and complement receptors 1 and 3. The microbicidal effect of neutrophils is due to a large repertoire of molecules present in pre-formed granules. Enzymes and other molecules prepared in these granules are proteases, such as collagenase, gelatinase or serine proteases, myeloperoxidase, lactoferrin and antibiotic proteins. Degranulation of these into the phagosome, accompanied by high reactive oxygen species production (oxidative burst) is highly microbicidal.[12]
Monocytes, and the macrophages that mature from them, leave blood circulation to migrate through tissues. There they are resident cells and form a resting barrier.[11] Macrophages initiate phagocytosis by mannose receptors, scavenger receptors, Fcγ receptors and complement receptors 1, 3 and 4. Macrophages are long-lived and can continue phagocytosis by forming new lysosomes.[11][13]
Dendritic cells also reside in tissues and ingest pathogens by phagocytosis. Their role is not killing or clearance of microbes, but rather breaking them down for antigen presentation to the cells of the adaptive immune system.[11]
Initiating receptors
Receptors for phagocytosis can be divided into two categories by recognised molecules. The first, opsonic receptors, are dependent on
Fcγ receptors
Fcγ receptors recognise IgG coated targets. The main recognised part is the
Complement receptors
These receptors recognise targets coated in
Mannose receptors
Mannose and other pathogen-associated sugars, such as fucose, are recognised by the mannose receptor. Eight lectin-like domains form the extracellular part of the receptor. The ingestion mediated by the mannose receptor is distinct in molecular mechanisms from Fcγ receptor or complement receptor mediated phagocytosis.[13]
Phagosome
Engulfment of material is facilitated by the actin-myosin contractile system. The phagosome is the organelle formed by phagocytosis of material. It then moves toward the centrosome of the phagocyte and is fused with lysosomes, forming a phagolysosome and leading to degradation. Progressively, the phagolysosome is acidified, activating degradative enzymes.[10][15]
Degradation can be oxygen-dependent or oxygen-independent.
- Oxygen-dependent degradation depends on NADPH and the production of reactive oxygen species. Hydrogen peroxide and myeloperoxidase activate a halogenating system, which leads to the creation of hypochlorite and the destruction of bacteria.[16]
- Oxygen-independent degradation depends on the release of granules, containing enzymes such as lysozymes, and cationic proteins such as defensins. Other antimicrobial peptides are present in these granules, including lactoferrin, which sequesters iron to provide unfavourable growth conditions for bacteria. Other enzymes like hyaluronidase, lipase, collagenase, elastase, ribonuclease, deoxyribonuclease also play an important role in preventing the spread of infection and degradation of essential microbial biomolecules leading to cell death.[12][13]
Some bacteria, for example
In apoptosis
Following
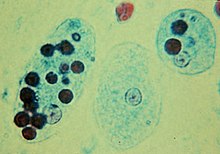
In protists
In many protists, phagocytosis is used as a means of feeding, providing part or all of their nourishment. This is called phagotrophic nutrition, distinguished from osmotrophic nutrition which takes place by absorption.[citation needed]
- In some, such as pseudopods, as in animal phagocytes. In humans, the amoebozoan Entamoeba histolytica can phagocytose red blood cells.
- Ciliates also engage in phagocytosis.[25] In ciliates there is a specialized groove or chamber in the cell where phagocytosis takes place, called the cytostome or mouth.
As in phagocytic immune cells, the resulting phagosome may be merged with lysosomes(food vacuoles) containing digestive enzymes, forming a phagolysosome. The food particles will then be digested, and the released nutrients are diffused or transported into the cytosol for use in other metabolic processes.[26]
See also
References
- PMID 1733516.
- PMID 27227301.
- ISBN 978-1-55938-999-0, retrieved 2023-04-06
- S2CID 218618570.
- ^ ISSN 0003-1569.
- ISBN 978-1-4615-7301-2, retrieved 2023-04-07
- ^ S2CID 39306211.
- OCLC 1042894741. Retrieved 2023-04-07.
- PMID 16876776.
- ^ PMID 26982354.
- ^ )
- ^ PMID 10830774.
- ^ PMID 10358769.
- ^ The Immune System, Peter Parham, Garland Science, 2nd edition
- PMID 21910624.
- PMID 1547201.
- S2CID 12277593.
- PMID 2989021.
- ISBN 978-1-4684-5498-7.
- PMID 22074924.
- PMID 19838202.
- PMID 21135166.
- S2CID 12729066.
- PMID 22196723.
- PMID 12089212.
- ISSN 0948-3055.
- PMID 12812372.
External links
 Media related to Phagocytosis at Wikimedia Commons
Media related to Phagocytosis at Wikimedia Commons- Phagocytosis at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
