Phagocyte
Phagocytes are
Phagocytes of humans and other animals are called "professional" or "non-professional" depending on how effective they are at phagocytosis.[7] The professional phagocytes include many types of white blood cells (such as neutrophils, monocytes, macrophages, mast cells, and dendritic cells).[8] The main difference between professional and non-professional phagocytes is that the professional phagocytes have molecules called receptors on their surfaces that can detect harmful objects, such as bacteria, that are not normally found in the body. Non-professional phagocytes do not have efficient phagocytic receptors, such as those for opsonins.[9] Phagocytes are crucial in fighting infections, as well as in maintaining healthy tissues by removing dead and dying cells that have reached the end of their lifespan.[10]
During an infection, chemical signals attract phagocytes to places where the pathogen has invaded the body. These chemicals may come from bacteria or from other phagocytes already present. The phagocytes move by a method called
History

The Russian zoologist
A year later, Mechnikov studied a fresh water crustacean called Daphnia, a tiny transparent animal that can be examined directly under a microscope. He discovered that fungal spores that attacked the animal were destroyed by phagocytes. He went on to extend his observations to the white blood cells of mammals and discovered that the bacterium Bacillus anthracis could be engulfed and killed by phagocytes, a process that he called phagocytosis.[18] Mechnikov proposed that phagocytes were a primary defense against invading organisms.[15]
In 1903,
Although the importance of these discoveries slowly gained acceptance during the early twentieth century, the intricate relationships between phagocytes and all the other components of the immune system were not known until the 1980s.[20]
Phagocytosis
![A cartoon: 1. The particle is depicted by an oval and the surface of the phagocyte by a straight line. Different smaller shapes are on the line and the oval. 2. The smaller particles on each surface join. 3. The line is now concave and partially wraps around the oval.[21]](http://upload.wikimedia.org/wikipedia/commons/thumb/b/ba/Phagocytosis_in_three_steps.png/250px-Phagocytosis_in_three_steps.png)
Phagocytosis is the process of taking in particles such as bacteria, invasive

A phagocyte has many types of receptors on its surface that are used to bind material.
Methods of killing

The killing of microbes is a critical function of phagocytes that is performed either within the phagocyte (
Oxygen-dependent intracellular
When a phagocyte ingests bacteria (or any material), its oxygen consumption increases. The increase in oxygen consumption, called a respiratory burst, produces reactive oxygen-containing molecules that are anti-microbial.[32] The oxygen compounds are toxic to both the invader and the cell itself, so they are kept in compartments inside the cell. This method of killing invading microbes by using the reactive oxygen-containing molecules is referred to as oxygen-dependent intracellular killing, of which there are two types.[12]
The first type is the oxygen-dependent production of a
The second type involves the use of the enzyme myeloperoxidase from neutrophil granules.[34] When granules fuse with a phagosome, myeloperoxidase is released into the phagolysosome, and this enzyme uses hydrogen peroxide and chlorine to create hypochlorite, a substance used in domestic bleach. Hypochlorite is extremely toxic to bacteria.[14] Myeloperoxidase contains a heme pigment, which accounts for the green color of secretions rich in neutrophils, such as pus and infected sputum.[35]
Oxygen-independent intracellular
Phagocytes can also kill microbes by oxygen-independent methods, but these are not as effective as the oxygen-dependent ones. There are four main types. The first uses electrically charged proteins that damage the bacterium's
Extracellular
In some diseases, e.g., the rare chronic granulomatous disease, the efficiency of phagocytes is impaired, and recurrent bacterial infections are a problem.[41] In this disease there is an abnormality affecting different elements of oxygen-dependent killing. Other rare congenital abnormalities, such as Chédiak–Higashi syndrome, are also associated with defective killing of ingested microbes.[42]
Viruses
Viruses can reproduce only inside cells, and they can gain entry by using many of the receptors involved in immunity. Once inside the cell, viruses use the cell's biological machinery to their own advantage, forcing the cell to make hundreds of identical copies of themselves. Although phagocytes and other components of the innate immune system can, to a limited extent, control viruses, once a virus is inside a cell the adaptive immune responses, particularly the lymphocytes, are more important for defense.[43] At the sites of viral infections, lymphocytes often vastly outnumber all the other cells of the immune system; this is common in viral meningitis.[44] Virus-infected cells that have been killed by lymphocytes are cleared from the body by phagocytes.[45]
Role in apoptosis
In an animal, cells are constantly dying. A balance between cell division and cell death keeps the number of cells relatively constant in adults.[10] There are two different ways a cell can die: by necrosis or by apoptosis. In contrast to necrosis, which often results from disease or trauma, apoptosis—or programmed cell death—is a normal healthy function of cells. The body has to rid itself of millions of dead or dying cells every day, and phagocytes play a crucial role in this process.[46]
Dying cells that undergo the final stages of
Interactions with other cells
Phagocytes are usually not bound to any particular
Antigen presentation

Antigen presentation is a process in which some phagocytes move parts of engulfed materials back to the surface of their cells and "present" them to other cells of the immune system.[57] There are two "professional" antigen-presenting cells: macrophages and dendritic cells.[58] After engulfment, foreign proteins (the antigens) are broken down into peptides inside dendritic cells and macrophages. These peptides are then bound to the cell's major histocompatibility complex (MHC) glycoproteins, which carry the peptides back to the phagocyte's surface where they can be "presented" to lymphocytes.[13] Mature macrophages do not travel far from the site of infection, but dendritic cells can reach the body's lymph nodes, where there are millions of lymphocytes.[59] This enhances immunity because the lymphocytes respond to the antigens presented by the dendritic cells just as they would at the site of the original infection.[60] But dendritic cells can also destroy or pacify lymphocytes if they recognize components of the host body; this is necessary to prevent autoimmune reactions. This process is called tolerance.[61]
Immunological tolerance
Dendritic cells also promote immunological tolerance,[62] which stops the body from attacking itself. The first type of tolerance is central tolerance, that occurs in the thymus. T cells that bind (via their T cell receptor) to self antigen (presented by dendritic cells on MHC molecules) too strongly are induced to die. The second type of immunological tolerance is peripheral tolerance. Some self reactive T cells escape the thymus for a number of reasons, mainly due to the lack of expression of some self antigens in the thymus. Another type of T cell; T regulatory cells can down regulate self reactive T cells in the periphery.[63] When immunological tolerance fails, autoimmune diseases can follow.[64]
Professional phagocytes

Phagocytes of humans and other jawed vertebrates are divided into "professional" and "non-professional" groups based on the efficiency with which they participate in phagocytosis.
Activation
All phagocytes, and especially macrophages, exist in degrees of readiness. Macrophages are usually relatively dormant in the tissues and proliferate slowly. In this semi-resting state, they clear away dead host cells and other non-infectious debris and rarely take part in antigen presentation. But, during an infection, they receive chemical signals—usually interferon gamma—which increases their production of MHC II molecules and which prepares them for presenting antigens. In this state, macrophages are good antigen presenters and killers. If they receive a signal directly from an invader, they become "hyperactivated", stop proliferating, and concentrate on killing. Their size and rate of phagocytosis increases—some become large enough to engulf invading protozoa.[65]
In the blood, neutrophils are inactive but are swept along at high speed. When they receive signals from macrophages at the sites of inflammation, they slow down and leave the blood. In the tissues, they are activated by cytokines and arrive at the battle scene ready to kill.[66]
Migration

When an infection occurs, a chemical "SOS" signal is given off to attract phagocytes to the site.
To reach the site of infection, phagocytes leave the bloodstream and enter the affected tissues. Signals from the infection cause the
Monocytes
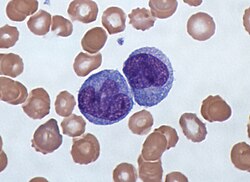
Monocytes develop in the bone marrow and reach maturity in the blood. Mature monocytes have large, smooth, lobed nuclei and abundant
Macrophages
Mature macrophages do not travel far but stand guard over those areas of the body that are exposed to the outside world. There they act as garbage collectors, antigen presenting cells, or ferocious killers, depending on the signals they receive.[72] They derive from monocytes, granulocyte stem cells, or the cell division of pre-existing macrophages.[73] Human macrophages are about 21 micrometers in diameter.[74]

This type of phagocyte does not have granules but contains many
Macrophages can be activated to perform functions that a resting monocyte cannot.
Neutrophils
Neutrophils are normally found in the bloodstream and are the most abundant type of phagocyte, constituting 50% to 60% of the total circulating white blood cells.[79] One litre of human blood contains about five billion neutrophils,[3] which are about 10 micrometers in diameter[80] and live for only about five days.[40] Once they have received the appropriate signals, it takes them about thirty minutes to leave the blood and reach the site of an infection.[81] They are ferocious eaters and rapidly engulf invaders coated with antibodies and complement, and damaged cells or cellular debris. Neutrophils do not return to the blood; they turn into pus cells and die.[81] Mature neutrophils are smaller than monocytes and have a segmented nucleus with several sections; each section is connected by chromatin filaments—neutrophils can have 2–5 segments. Neutrophils do not normally exit the bone marrow until maturity but during an infection neutrophil precursors called metamyelocytes, myelocytes and promyelocytes are released.[82]
The intra-cellular granules of the human neutrophil have long been recognized for their protein-destroying and bactericidal properties.
Dendritic cells

Dendritic cells are specialized antigen-presenting cells that have long outgrowths called dendrites,
Mast cells
Mast cells have
| Main location | Variety of phenotypes |
|---|---|
| Blood | neutrophils, monocytes |
| Bone marrow | macrophages, monocytes, lining cells
|
| Bone tissue | osteoclasts |
| Gut and intestinal Peyer's patches
|
macrophages |
| Connective tissue | histiocytes, macrophages, monocytes, dendritic cells |
| Liver | Kupffer cells, monocytes |
| Lung | self-replicating macrophages, monocytes, mast cells, dendritic cells |
| Lymphoid tissue | free and fixed macrophages and monocytes, dendritic cells |
| Nervous tissue | microglial cells (CD4 +)
|
| Spleen | free and fixed macrophages, monocytes, sinusoidal cells |
| Thymus | free and fixed macrophages and monocytes |
| Skin | resident Langerhans cells, other dendritic cells, conventional macrophages, mast cells |
Non-professional phagocytes
Dying cells and foreign organisms are consumed by cells other than the "professional" phagocytes.
Non-professional phagocytes are more limited than professional phagocytes in the type of particles they can take up. This is due to their lack of efficient phagocytic receptors, in particular opsonins—which are antibodies and complement attached to invaders by the immune system.[9] Additionally, most non-professional phagocytes do not produce reactive oxygen-containing molecules in response to phagocytosis.[104]
| Main location | Variety of phenotypes |
|---|---|
| Blood, lymph and lymph nodes | Lymphocytes |
| Blood, lymph and lymph nodes | NK and LGL cells (large granular lymphocytes)
|
| Blood | Basophils[105]
|
| Skin | Epithelial cells
|
| Liver | Hepatocytes[106] |
| Blood vessels | Endothelial cells
|
| Connective tissue | Fibroblasts |
Pathogen evasion and resistance
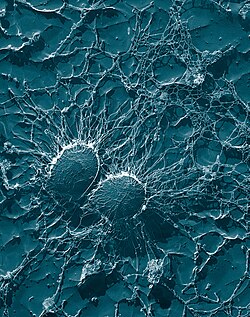
A pathogen is only successful in infecting an organism if it can get past its defenses. Pathogenic bacteria and protozoa have developed a variety of methods to resist attacks by phagocytes, and many actually survive and replicate within phagocytic cells.[107][108]
Avoiding contact
There are several ways bacteria avoid contact with phagocytes. First, they can grow in sites that phagocytes are not capable of traveling to (e.g., the surface of unbroken skin). Second, bacteria can suppress the
Avoiding engulfment
Bacteria often produce
Survival inside the phagocyte
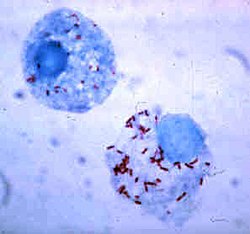
Bacteria have developed ways to survive inside phagocytes, where they continue to evade the immune system.
Killing
Bacteria have developed several ways of killing phagocytes.
Disruption of cell signaling
Some survival strategies often involve disrupting cytokines and other methods of cell signaling to prevent the phagocyte's responding to invasion.[124] The protozoan parasites Toxoplasma gondii, Trypanosoma cruzi, and Leishmania infect macrophages, and each has a unique way of taming them.[124] Some species of Leishmania alter the infected macrophage's signalling, repress the production of cytokines and microbicidal molecules—nitric oxide and reactive oxygen species—and compromise antigen presentation.[125]
Host damage by phagocytes
Macrophages and neutrophils, in particular, play a central role in the inflammatory process by releasing proteins and small-molecule inflammatory mediators that control infection but can damage host tissue. In general, phagocytes aim to destroy pathogens by engulfing them and subjecting them to a battery of toxic chemicals inside a phagolysosome. If a phagocyte fails to engulf its target, these toxic agents can be released into the environment (an action referred to as "frustrated phagocytosis"). As these agents are also toxic to host cells, they can cause extensive damage to healthy cells and tissues.[126]
When neutrophils release their granule contents in the
Neutrophils also play a key role in the development of most forms of
Chemicals released by macrophages can also damage host tissue.
Evolutionary origins

Phagocytosis is common and probably appeared early in
References
- ^ a b Delves et al. 2006, p. 250
- ^ Delves et al. 2006, p. 251
- ^ a b c d Hoffbrand, Pettit & Moss 2005, p. 331
- ^ Ilya Mechnikov, retrieved on November 28, 2008. From Nobel Lectures, Physiology or Medicine 1901–1921, Elsevier Publishing Company, Amsterdam, 1967. Archived August 22, 2008, at the Wayback Machine
- ^ S2CID 25063709.
- ^ a b Janeway, Chapter: Evolution of the innate immune system. retrieved on March 20, 2009
- ^ a b Ernst & Stendahl 2006, p. 186
- ^ a b Robinson & Babcock 1998, p. 187 and Ernst & Stendahl 2006, pp. 7–10
- ^ a b Ernst & Stendahl 2006, p. 10
- ^ S2CID 12991980.
- ^ a b c Janeway, Chapter: Induced innate responses to infection.
- ^ S2CID 11063073.
- ^ a b Delves et al. 2006, pp. 172–84
- ^ a b c d e f g h i Delves et al. 2006, pp. 2–10
- ^ PMID 31001278.
- ^ Little C, Fowler HW, Coulson J (1983). The Shorter Oxford English Dictionary. Oxford University Press (Guild Publishing). pp. 1566–67.
- S2CID 44748502.
- ^ "Ilya Mechnikov". The Nobel Foundation. Retrieved December 19, 2014.
- ^ Delves et al. 2006, p. 263
- ^ Robinson & Babcock 1998, p. vii
- ^ Ernst & Stendahl 2006, p. 6
- ^ Ernst & Stendahl 2006, p. 4
- ^ Ernst & Stendahl 2006, p. 78
- PMID 29727727.
- ^ S2CID 44911791. Archived from the originalon December 28, 2012. Retrieved December 19, 2014.
- ^ Delves et al. 2006, pp. 6–7
- ^ Sompayrac 2019, p. 2
- ^ Sompayrac 2019, p. 2
- ^ Sompayrac 2019, pp. 13–16
- PMID 30699960.
- S2CID 746699.
- PMID 10618505.
- PMID 9022278.
- PMID 10519157.
- PMID 15478278.
- ^ Hoffbrand, Pettit & Moss 2005, p. 118
- ^ Delves et al. 2006, pp. 6–10
- S2CID 15862242.
- ^ Delves et al. 2006, p. 188
- ^ a b Sompayrac 2019, p. 136
- PMID 18846805.
- S2CID 43243529.
- ^ Sompayrac 2019, p. 7
- PMID 17962876.
- ^ Sompayrac 2019, p. 22
- ^ Sompayrac 2019, p. 68
- ^ "Apoptosis". Merriam-Webster Online Dictionary. Retrieved December 19, 2014.
- S2CID 36252352. (Free registration required for online access)
- PMID 31837595.
- S2CID 25672278. Archived from the originalon April 14, 2021. (Free registration required for online access)
- S2CID 13402617.
- PMID 18774293.
- ^ Sompayrac 2019, p. 3
- ^ Sompayrac 2019, p. 4
- ^ Sompayrac 2019, pp. 27–35
- ^ Delves et al. 2006, pp. 171–184
- ^ Delves et al. 2006, pp. 456
- ^ Lee T, McGibbon A (2004). "Antigen Presenting Cells (APC)". Dalhousie University. Archived from the original on January 12, 2008. Retrieved December 19, 2014.
- ^ Delves et al. 2006, p. 161
- ^ Sompayrac 2019, p. 8
- ^ Delves et al. 2006, pp. 237–242
- S2CID 36342899.
- ^ Steinman, Ralph M. (2004). "Dendritic Cells and Immune Tolerance". The Rockefeller University. Archived from the original on March 11, 2009. Retrieved December 19, 2014.
- S2CID 27585046.
- ^ Sompayrac 2019, pp. 16–17
- ^ Sompayrac 2019, pp. 18–19
- ^ Delves et al. 2006, p. 6
- PMID 14519390.
- ^ Sompayrac 2019, p. 18
- ^ Hoffbrand, Pettit & Moss 2005, p. 117
- ^ Delves et al. 2006, pp. 1–6
- ^ Sompayrac 2019, p. 136
- S2CID 6049656.
- PMID 9400735.
- ^ a b c d e Delves et al. 2006, pp. 31–36
- ^ Ernst & Stendahl 2006, p. 8
- ^ Delves et al. 2006, p. 156
- ^ Delves et al. 2006, p. 187
- ISBN 978-80-967366-1-4. Archived from the originalon December 31, 2010. Retrieved December 19, 2014.
- ^ Delves et al. 2006, p. 4
- ^ a b Sompayrac 2019, p. 18
- PMID 9853933.
- ^ Paoletti, Notario & Ricevuti 1997, p. 62
- PMID 17991288.
- PMID 18787642.
- S2CID 25067858.
- PMID 4573839.
- ^ a b Steinman, Ralph. "Dendritic Cells". The Rockefeller University. Archived from the original on June 27, 2009. Retrieved December 19, 2014.
- PMID 11861614.
- ^ Hoffbrand, Pettit & Moss 2005, p. 134
- PMID 12110131.
- ^ Sompayrac 2019, pp. 45–46
- PMID 19672091. Retrieved December 19, 2014.
- PMID 18936782.
- ^ S2CID 23115222.
- PMID 8790416.
- S2CID 7917861. Retrieved December 19, 2014.
- PMID 11424870.
- PMID 22577358.
- ^ a b Paoletti, Notario & Ricevuti 1997, p. 427
- PMID 18451871.
- PMID 11083817.
- PMID 11112696.
- PMID 14732160.
- PMID 29321780.
- PMID 32528462.
- ^ a b c d e Todar, Kenneth. "Mechanisms of Bacterial Pathogenicity: Bacterial Defense Against Phagocytes". 2008. Retrieved December 19, 2014.
- PMID 10462516. Retrieved December 19, 2014.
- PMID 11973157.
- PMID 15992798.
- PMID 10417134.
- PMID 15501828.
- PMID 19047408.
- ^ S2CID 205496221.
- PMID 11890550.
- PMID 11708894.
- PMID 10064587.
- PMID 9807783.
- S2CID 22127111.
- PMID 17517863.
- ^ PMID 35003097.
- S2CID 14748436.
- PMID 16679003.
- ^ PMID 15639739.
- S2CID 24696519.
- ^ Paoletti pp. 426–30
- PMID 10430993.
- S2CID 24164360.
- ^ S2CID 29374195.
- S2CID 4004607.
- S2CID 10318084.
- PMID 17135502.
- ^ Sompayrac 2019, p. 2
- ^ PMID 18550419.
- S2CID 7326149.
- PMID 17673666.
- ^ Delves et al. 2006, pp. 251–252
- PMID 19063916.
Bibliography
- Delves, P. J.; Martin, S. J.; Burton, D. R.; Roit, I. M. (2006). Roitt's Essential Immunology (11th ed.). Malden, MA: Blackwell Publishing. ISBN 978-1-4051-3603-7.
- Ernst, J. D.; Stendahl, O., eds. (2006). Phagocytosis of Bacteria and Bacterial Pathogenicity. New York: Cambridge University Press. ISBN 978-0-521-84569-4. Website
- Hoffbrand, A. V.; Pettit, J. E.; Moss, P. A. H. (2005). Essential Haematology (4th ed.). London: Blackwell Science. ISBN 978-0-632-05153-3.
- Paoletti, R.; Notario, A.; Ricevuti, G., eds. (1997). Phagocytes: Biology, Physiology, Pathology, and Pharmacotherapeutics. New York: The New York Academy of Sciences. ISBN 978-1-57331-102-1.
- Robinson, J. P.; Babcock, G. F., eds. (1998). Phagocyte Function — A guide for research and clinical evaluation. New York: Wiley–Liss. ISBN 978-0-471-12364-4.
- Sompayrac, L. (2019). How the Immune System Works (6th ed.). Malden, MA: Blackwell Publishing. ISBN 978-1-119-54212-4.
External links
- Phagocytes at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- White blood cell engulfing bacteria
