Sandwich compound

In
The term sandwich compound was introduced in organometallic nomenclature in 1956 in a report by J. D. Dunitz, L. E. Orgel and R. A. Rich, who confirmed the structure of ferrocene by X-ray crystallography.[1] The correct structure, in which the molecule features an iron atom sandwiched between two parallel cyclopentadienyl rings, had been proposed several years previously by Robert Burns Woodward and, separately, by Ernst Otto Fischer. The structure helped explain puzzles about ferrocene's conformers. This result further demonstrated the power of X-ray crystallography and accelerated the growth of organometallic chemistry.[2][page needed]
Classes
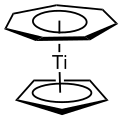
(Cycloheptatrienyl)(cyclopentadienyl)titanium (troticene) is an unsymmetrical sandwich complex.[3]
The best known members are the metallocenes of the formula M(C5H5)2 where M = Cr, Fe, Co, Ni, Pb, Zr, Ru, Rh, Os, Sm, Ti, V, Mo, W, Zn. These species are also called bis(cyclopentadienyl)metal complexes. Other arenes can serve as ligands as well.
- Mixed cyclopentadienyl complexes: M(C5H5)(CnHn). Some examples are Ti(C5H5)(C7H7) and (C60)Fe(C5H5Ph5) where the fullerene ligandis acting as a cyclopentadienyl analogue.
- Bis(benzene) complexes: M(C6H6)2, the best known example being bis(benzene)chromium.
- Bis(cyclooctatetraenyl) complexes: M(C8H8)2, such as U(C8H8)2 and Th(C8H8)2 (both actinocenes).
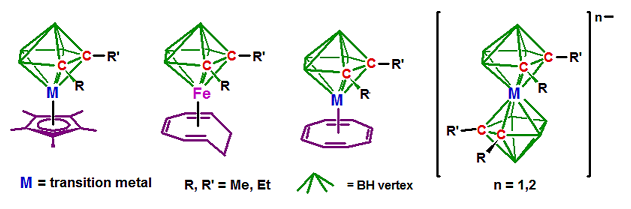
- Metal–polyhedral cages ranging in size from 6 to 15 vertices. Examples include bis(dicarbollide) complexes,[4] such as [M(C2B9H11)2]z− and [Fe(C2B9H11)2]2−, and small-carborane sandwiches such as (R2C2B3H5)M(C2B4H6) and (R5C5)M(R′2)C2B4H4) where M is a transition metal and R and R′ are methyl or ethyl.[5][6]

Closely related are the metal complexes containing H3C3B2R2 (diborolyl) ligands.[7] In addition to these, other sandwich complexes containing purely inorganic ligands are known, such as Fe(C5Me5)(P5) and [(P5)2Ti]2−.[8]
Half-sandwich compounds
Monometallic half-sandwich compounds

Dimetallic half-sandwich
Compounds such as the
Multidecker sandwiches
The first isolated multidecker sandwich was the tris(cyclopentadienyl)dinickel triple-decker complex [Ni2Cp3]BF4, a highly air- and water-sensitive compound reported in 1972,[11] with X-ray crystallographic confirmation in 1974.[12]
In 1973 the electrically neutral air-stable triple-decker cobaltacarborane sandwiches 1,7,2,3- and 1,7,2,4-CpCo(RHC2B3H3)Cp (where R = H, Me) were isolated and characterized by multinuclear NMR and X-ray studies[13] (the structure of the 1,7,2,3 isomer is shown).

Since then many three-, four-, five-, and six-decker sandwich complexes have been described.[14][15] The largest structurally characterized multidecker sandwich monomer is the hexadecker shown at lower right.[16]

An extensive family of multidecker sandwiches incorporating planar (R2R′C3B2R″2)3− (diborolyl) ligands has also been prepared.[17]
Numerous multidecker sandwich compounds featuring hydrocarbon bridging rings have also been prepared, especially triple deckers.[18] A versatile method involves the attachment of Cp*Ru+ to preformed sandwich complexes.[19]
Linked sandwiches
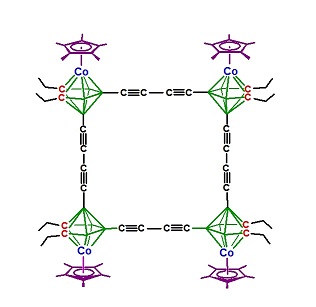
Monomeric double-decker and multidecker sandwiches have been used as building blocks for extended systems, some of which exhibit electron delocalization between metal centers. An example of a cyclic poly(metallacarborane) complex is the octahedral "carbon-wired" system shown below, which contains a planar C16B8 macrocycle.[20]
Inverse sandwiches
In these anti-bimetallic compounds, the metals are found to be bridged by a single carbocyclic ring. Examples include [(THF)3Ca]2(1,3,5-triphenylbenzene)[21] and [(Ar)Sn]2COT.
Double- and multimetallic sandwich compounds
Another family of sandwich compound involves more than one metal sandwiched between two carbocyclic rings. Examples of the double sandwich include V2(
Applications
Ferrocene and
References
- .
- ISBN 0-13-035471-6.
- .
- ^ .
- .
- ISBN 9780128019054.
- .
- S2CID 36455193.
- ^ .
- .
- .
- .
- ^ .
- ISBN 978-0-08-045047-6.
- .
- ^ .
- ISBN 9780120311354.
- .
- .
- PMID 12616549.
- PMID 19193100.
- .
- .
- PMID 12848540.
- .