Cobalamin biosynthesis

Cobalamin biosynthesis is the process by which
The feature which distinguishes the two main
Many prokaryotic species cannot biosynthesize adenosylcobalamin, but can make it from cobalamin which they assimilate from external sources. In humans, dietary sources of cobalamin are bound after ingestion as transcobalamins and converted to the coenzyme forms in which they are used.
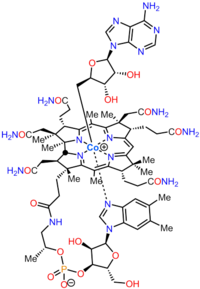
Cobalamin
Cobalamin (vitamin B12) is the largest and most structurally complex
Overview of cobalamin biosynthesis
There are at least two distinct cobalamin biosynthetic pathways in
 |
 |
- Aerobic pathway that requires oxygen and in which cobalt is inserted late in the pathway;[6][7] found in Pseudomonas denitrificans and Rhodobacter capsulatus.
- Salmonella typhimurium, Bacillus megaterium, and Propionibacterium freudenreichii subsp. shermanii.
Either pathway can be divided into two parts:
- Corrin ring synthesis leading to cobyrinic acid, with seven carboxylate groups. In the anaerobic pathway this already contains cobalt but in the aerobic pathway the material formed at that stage is hydrogenobyrinic acid, without the bound cobalt.[11][12][5]
- Insertion of cobalt, where not already present; formation of amides on all but one of the carboxylate groups to give cobyric acid; attachment of an adenosyl group as ligand to the cobalt; attachment of an aminopropanol sidechain to the one free carboxylic group and assembly of the nucleotide loop which will provide the second ligand for the cobalt.[5][13]
A further type of synthesis occurs through a
Detail of steps up to formation of uroporphyrinogen III
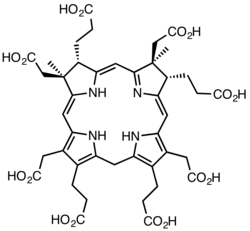
In the early steps of the biosynthesis, a tetrapyrrolic structural framework is created by the enzymes deaminase and cosynthetase which transform aminolevulinic acid via porphobilinogen and hydroxymethylbilane to uroporphyrinogen III. The latter is the first macrocyclic intermediate common to haem, chlorophyll, sirohaem and cobalamin itself.[7][16][17]
Detail of steps from uroporphyrinogen III to cob(II)yrinic acid a,c-diamide in aerobic organisms
The biosynthesis of cobalamin diverges from that of haem and chlorophyll at uroporphrinogen III: its transformation involves the sequential addition of methyl (CH3) groups to give intermediates that were given
From uroporphyrinogen III to precorrin-2
The enzyme CobA catalyses two methylations, to give precorrin-2:[20]
- (1a) uroporphyrinogen III + S-adenosyl methionineprecorrin-1 +S-adenosyl-L-homocysteine
- (1b) precorrin-1 + S-adenosyl methionine precorrin-2 + S-adenosyl-L-homocysteine
From precorrin-2 to precorrin-3A
The enzyme CobI then converts this to precorrin-3A:[18]
- precorrin-2 + S-adenosyl methionine precorrin-3A + S-adenosyl-L-homocysteine
From precorrin-3A to precorrin-3B
Next, the enzyme CobG transforms precorrin-3A to precorrin-3B:[18]
- precorrin-3A + NADH + H+ + O2 precorrin-3B + NAD+ + H2O
This enzyme is an oxidoreductase that requires oxygen and hence the reaction can only operate under aerobic conditions. The naming of these precorrins as 3A and 3B reflects the fact that each contains three more methyl groups than uroporphyrinogen III but with different structures: in particular, precorrin-3B has an internal γ-lactone ring formed from the ring A acetic acid sidechain closing back on to the macrocycle.
From precorrin-3B to precorrin-4
The enzyme
From precorrin-4 to precorrin-5
Methyl group insertions continue as the enzyme CobM acts on precorrin-4:[21]
- precorrin-4 + S-adenosyl methionine precorrin-5 + S-adenosyl-L-homocysteine
The newly-inserted methyl group is added to ring C at the carbon attached to the methylene (CH2) bridge to ring B. This is not its final location on cobalamin as a later step involves its rearrangement to an adjacent ring carbon.
From precorrin-5 to precorrin-6A
The enzyme CobF now removes the acetyl group located at position 1 of the ring system in precorrin-4 and replaces it with a newly-introduced methyl group. The name of the product, precorrin-6A, reflects the fact that six methyl groups in total have been added to uroporphyrinogen III up to this point. However, since one of these has been extruded with the acetate group, the structure of precorrin-6A contains just the remaining five.[21]
From precorrin-6A to precorrin-6B
The enzyme
- precorrin-6A + NADPH + H+ precorrin-6B + NADP+
Precorrin-6B therefore differs in structure from precorrin-6A only by having an extra two hydrogen atoms.
From precorrin-6B to precorrin-8
The enzyme CobL has two active sites, one catalysing two methyl group additions and the other the decarboxylation of the CH2COOH group on ring D, so that this substituent becomes a simple methyl group:[21]
From precorrin-8 to hydrogenobyrinic acid
The enzyme CobH catalyzes a rearrangement reaction, with the result that the methyl group that had been added to ring C is isomerised to its final location, an example of intramolecular transfer:[22]
- precorrin-8X hydrogenobyrinate
From hydrogenobyrinic acid to hydrogenobyrinic acid a,c-diamide
The next enzyme in the pathway, CobB, selectively converts two of the eight carboxylic acid groups into their primary amides. ATP is used to provide the energy for amide bond formation, with the transferred ammonia coming from glutamine:[23]
- hydrogenobyrinic acid + 2 ATP + 2 glutamine + 2 H2O hydrogenobyrinic acid a,c-diamide + 2 ADP + 2 phosphate + 2 glutamic acid
From hydrogenobyrinic acid a,c-diamide to cob(II)yrinic acid a,c-diamide
Cobalt(II) insertion into the macrocycle is catalysed by the enzyme Cobalt chelatase (CobNST):[24]
- hydrogenobyrinic acid a,c-diamide + Co2+ + ATP + H2O cob(II)yrinic acid a,c-diamide + ADP + phosphate + H+
It is at this stage that the aerobic pathway and the anaerobic pathway merge, with later steps being chemically identical.
Detail of steps from uroporphyrinogen III to cob(II)yrinic acid a,c-diamide in anaerobic organisms
Many of the steps beyond uroporphyrinogen III in anaerobic organisms such as Bacillus megaterium involve chemically similar but genetically distinct transformations to those in the aerobic pathway.[10][25]
From precorrin-2 to cobalt-sirohydrochlorin
The key difference in the pathways is that cobalt is inserted early in anaerobic organisms by first oxidising precorrin-2 to its fully aromatised form sirohydrochlorin and then to that compound's cobalt(II) complex.[26] These reactions are catalysed by CysG and Sirohydrochlorin cobaltochelatase.[27]
From cobalt-sirohydrochlorin to cobalt-factor III
As in the aerobic pathway, the third methyl group is introduced by a methyltransferase enzyme, CbiL:[26]
- cobalt-sirohydrochlorin + S-adenosyl methionine cobalt-factor III + S-adenosyl-L-homocysteine
From cobalt-factor III to cobalt-precorrin-4
Methylation and ring contraction to form the corrin macrocycle occurs next, catalysed by the enzyme Cobalt-factor III methyltransferase (CbiH, EC 2.1.1.272)[28]
In this pathway, the resulting material contains a δ-lactone, a six-membered ring, rather than the γ-lactone (five-membered ring) of precorrin-3B.
From cobalt-precorrin-4 to cobalt-precorrin-5A
The introduction of the methyl group at C-11 in the next step is catalysed by Cobalt-precorrin-4 methyltransferase (CbiF, EC 2.1.1.271)[29]
- cobalt-precorrin-4 + S-adenosyl methionine cobalt-precorrin-5 + S-adenosyl-L-homocysteine
From cobalt-precorrin-5A to cobalt-precorrin-5B
The scene is now set for the extrusion of the two-carbon fragment corresponding to the acetate released in the formation of precorrin-6A in the aerobic pathway. In this case the fragment released is acetaldehyde and this is catalysed by CbiG:[29]
- cobalt-precorrin-5A + H2O cobalt-precorrin-5B + acetaldehyde + 2 H+
From cobalt-precorrin-5B to cob(II)yrinic acid a,c-diamide
The steps from cobalt-precorrin-5B to cob(II)yrinic acid a,c-diamide in the anaerobic pathway are essentially chemically identical to those in the aerobic sequence. The intermediates are called cobalt-precorrin-6A, cobalt-precorrin-6B, cobalt-precorrin-8 and cobyrinic acid. The enzymes in sequence are CbiD;[30] Cobalt-precorrin-6A reductase (CbiJ, EC 1.3.1.106);[31] CbiT, Cobalt-precorrin-8 methylmutase (CbiC, EC 5.4.99.60) and CbiA. The final enzyme forms cob(II)yrinic acid a,c-diamide as the two pathways converge.[5]
Detail of steps from cob(II)yrinic acid a,c-diamide to adenosylcobalamin
Aerobic and anaerobic organisms share the same chemical pathway beyond cob(II)yrinic acid a,c-diamide and this is illustrated for the cob gene products.
From cob(II)yrinic acid a,c-diamide to adenosylcobyric acid
The cobalt(II) is reduced to cobalt(I) by the enzyme CobR and then the enzyme CobO attaches an adenosyl ligand to the metal. Next, the enzyme CobQ converts all the carboxylic acids, except the propionic acid on ring D, to their primary amides.[7][21]
From adenosylcobyric acid to adenosylcobinamide phosphate
In aerobic organisms, the enzyme
From adenosylcobinamide phosphate to adenosylcobalamin
In a separate branch of the pathway,
- Adenosylcobalamin-5'-phosphate + H2O adenosylcobalamin + phosphate
The complete biosynthetic route involves a long linear path that requires about 25 contributing enzyme steps.
Other pathways of cobalamin metabolism
Salvage pathways in prokaryotes
Many
Cobalamin metabolism in humans
In humans, dietary sources of cobalamin are bound after ingestion as transcobalamins.[39] They are then converted to the coenzyme forms in which they are used. Methylmalonic aciduria and homocystinuria type C protein is the enzyme which catalyzes the decyanation of cyanocobalamin as well as the dealkylation of alkylcobalamins including methylcobalamin and adenosylcobalamin.[40][41][42]
Further reading
- Layer G, Jahn D, Deery E, Lawrence AD, Warren MJ (2010). "Biosynthesis of Heme and Vitamin B12". Comprehensive Natural Products II. pp. 445–499. ISBN 9780080453828.
References
- PMID 12869542.
- PMID 17163662.
- ^ "Vitamin B12". Micronutrient Information Center, Linus Pauling Institute, Oregon State University, Corvallis, OR. 4 June 2015. Retrieved 20 April 2020.
- PMID 11153269.
- ^ PMID 28137297.
- S2CID 37362827.
- ^ a b c R. Caspi (2013-09-25). "Pathway: adenosylcobalamin biosynthesis II (aerobic)". MetaCyc Metabolic Pathway Database. Retrieved 2020-04-24.
- PMID 12055304.
- PMID 16042604.
- ^ a b R. Caspi (2013-09-25). "Pathway: adenosylcobalamin biosynthesis I (anaerobic)". MetaCyc Metabolic Pathway Database. Retrieved 2020-04-24.
- S2CID 83942303.
- PMID 11152419.
- S2CID 583311.
- PMID 13854292.
- S2CID 22232461.
- S2CID 9070849.
- PMID 16042604.
- ^ PMID 8226690.
- ISBN 0521828732.
- PMID 2407234.
- ^ PMID 12195810.
- PMID 1732194.
- PMID 2172209.
- PMID 1429466.
- PMID 16936030.
- ^ S2CID 26057998.
- S2CID 6613060.
- PMID 23155054.
- ^ PMID 16866557.
- PMID 15741157.
- PMID 16243778.
- ^ R. Caspi (2007-04-23). "Pathway: adenosylcobalamin biosynthesis from adenosylcobinamide-GDP I". MetaCyc Metabolic Pathway Database. Retrieved 2020-04-24.
- PMID 17209023.
- ^ R. Caspi (2013-09-25). "Pathway: adenosylcobalamin salvage from cobalamin". MetaCyc Metabolic Pathway Database. Retrieved 2020-04-24.
- PMID 2403541.
- PMID 14645280.
- PMID 14990804.
- PMID 16109931.
- ^ R. Caspi (2013-09-25). "Pathway: cobalamin salvage (eukaryotic)". MetaCyc Metabolic Pathway Database. Retrieved 2020-04-24.
- PMID 19447654.
- PMID 19665918.
- PMID 19832808.
External links
- Prof Sir Alan Battersby: the biosynthesis of Vitamin B12 St. Catharine's College, Cambridge, video






